��Ŀ����
����Cu����һ�ֽ�����������Mg��Fe��Zn�е�һ�֣��γɵķ�ĩ�������ⶨ����ɣ���������ʵ�飺ȡ�÷�ĩ16g�����ձ�����������������Ϊ14%��ϡ����280.0g��4�μ�����ձ��У���ַ�Ӧ���ʣ��Ĺ����������ݼ�¼���£�
| ʵ����� | 1 | 2 | 3 | 4 |
| ����ϡ����������g | 70.0 | 70.0 | 70.0 | 70.0 |
| ʣ�����������g | 13.6 | 11.2 | 8.8 | 8.4 |
���㣨Ҫ��д��������̣���
��1���÷�ĩ��Cu������������
��2���÷�ĩ����һ�ֽ���Ϊ���ֽ�����
��3����3�μ���ϡ�����ַ�Ӧ��������Һ�����ʵ�����������
��1��52.5 % ��2�֣�
��2���ý������ԭ������Ϊ 24 ���Խ���Ϊþ ��3�֣�
��3��16.6% ��3�֣�
���������������1��Cu���ܺ�ϡH2SO4��Ӧ��ʣ���������ΪCu��������
�÷�ĩ��Cu���������� =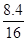 �� 100% = 52.5%
�� 100% = 52.5%
��2����÷�ĩ����һ�ֽ���Ϊ M���ý������ԭ������Ϊx
M + H2SO4 = MSO4 + H2 ��
X 98
2.4 70��14%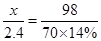
x =" 24"
�ý������ԭ������Ϊ 24 ���Խ���Ϊþ
��3���μӷ�Ӧ��þ������Ϊ7.6g ��������ҺΪ����þ��Һ����������þ��Һ����������������
�� þ��ϡ���ᷴӦ��������þ������Ϊy g ����������������Ϊ z g .
Mg + H2SO4 = MgSO4 + H2 ��
24 120 2
7.2 y z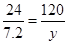
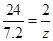
y = 36g z = 0.6g
��3�μ���ϡ�����ַ�Ӧ��������Һ�����ʵ���������= 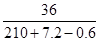 �� 100% = 16.6%
�� 100% = 16.6%
�� ����
���㣺���û�ѧ����ʽ���㡣

Cu��Zn�ĺϽ��Ϊ��ͭ���������ĵ����Ժ���ʴ�ԣ��������������������
��1���������ӳ��û�ͭ�Ƴɽ�������ġ���Ԫ������ƭ���ǣ����м����д�������� ����
| A������ɫ | B������ | C������������Һ | D�������� |
�ټ��װ�������Եķ����ǣ����Ӻ�װ�ã���©��עˮ�������������γɸ߶Ȳ���ñ�ǣ�һ��ʱ��߶Ȳ��� ����˵�����������á�
����ȷ��ȡ��������Һ��ij�ʼ�����ն���ǰ������еIJ������� �����۸�С�����������ʵ�飬������ص�ʵ�����ݴ������£�
| | �Ͻ������/g | ϡ��������/mL | ��������������/g |
| ��1�� | 2 | 15 | 0.04 |
| ��2�� | 2 | 20 | 0.04 |
| ��3�� | 4 | 15 | 0.04 |
b�������ϱ����ݣ����� ����ʵ�飬�Ͻ��е�п��ϡ����ǡ����ȫ��Ӧ��ϡ�����������������Ϊ�� �������������0.1%������ʵ���¶��£���(H2SO4)��1.08g/cm3��
 2CuSO4+2H2O������һ�������ʵ���������Ϊ9.8%��ϡ����ǡ�ô���2000g��ͭ3.2%�ķ��ϣ������������ʲ������ᷴӦ�Ҳ�����ˮ������Ӧ����������ͭ��Һ�����ʵ�����������
2CuSO4+2H2O������һ�������ʵ���������Ϊ9.8%��ϡ����ǡ�ô���2000g��ͭ3.2%�ķ��ϣ������������ʲ������ᷴӦ�Ҳ�����ˮ������Ӧ����������ͭ��Һ�����ʵ�����������