��Ŀ����
��Ԫ������������Ԫ�أ������ʳ������ȡ���㣬ҽ�����顰���ơ���
��1��ҽ�����顰���ơ���ָ������ �����Ԫ�ء����ʡ���ԭ�ӡ���
��2��̼�����̼Ԫ�صĻ��ϼ�Ϊ�� ����
��3��Ϊ�ⶨ�ò��Ƽ���̼��Ƶ������������ֳ�ȡ15�˵���Ʒ�����ձ�������м���������ϡ�����ַ�Ӧ�������ɷֲ�����Ԫ�ء�������ˮҲ����ϡ���ᷴӦ������������Ϊ4.4g��
���ж���ȡ��Ʒ����ȫ��Ӧ������Ϊ�� ����
�ڸò��Ƽ���Ʒ��̼��Ƶ���������Ϊ���٣������ü����������ʾ������������һλС����
��1��Ԫ�� ��2�� +4������3���������ݲ�����������������66.7%
���������������1��ҽ�����顰���ơ���ָ����Ԫ�أ�2��̼�����̼Ԫ�صĻ��ϼۣ����ݻ�������Ԫ�صĻ��ϼ۵Ĵ����Ͳ�Ϊ�㣬�ʿ�ȷ��̼Ԫ�صĻ��ϼ�Ϊ+4�ۣ���3�����ж���ȡ��Ʒ����ȫ��Ӧ������Ϊ�����ݲ�����������������
����μӷ�Ӧ��̼��Ƶ�����Ϊx
CaCO3 + 2HCl = CaCl2 + CO2��+ H2O
100 44
X 4.4g
100��44=X��4.4g
X=10g
�ò��Ƽ���Ʒ��̼��Ƶ���������Ϊ10 g /15 g��100%="66.7%"
�𣺸ò��Ƽ���Ʒ��̼��Ƶ���������Ϊ66.7%
���㣺���ϼ۵ļ��㡢���ݻ�ѧ����ʽ�ļ���.

ij������ÿ������4 000 t��Fe2O3 80%�ij�����ʯ���ø����������Ͽ��ղ���Fe 98%�����������Ƕ��٣�(������������0.1)
Ŀǰ�г��ϵIJ���ҩ���ܶ࣬��ͼ��ijƷ�Ʋ���ҩƷ�IJ���˵���顣Ϊ�ⶨ�京���Ƿ��꣬ij��ȤС���ͬѧȡ10Ƭ��Ƭ���������ձ��У���100gϡ�������μ��뵽�ձ��У������Ͻ��裬���������õ����й����ݼ�¼���£���Ƭ�������ɷֲ���ϡ���ᷴӦ����
| XXX��Ƭ [ҩƷ���]ÿƬ1g [ҩƷ����]��CaCO380% [�÷�����]ÿ��һƬ��ÿ��2�� |
| ����ϡ���������/g | 0 | 20 | 40 | 60 | 80 | 100 |
| �ձ���ʣ����������/g | 10 | 29��12 | 48��24 | 67��36 | 86��48 | 106��48 |
������������Ϣ�ͱ������ݣ��ش��������⣺
��1��ʵ������н���Ƭ�����Ŀ���ǣ� ��
��2����ȫ��Ӧ����������̼������Ϊ g
��3����ͨ������˵������Ʒ�Ƹ�Ƭ�����Ƿ��ꡣ
��4��������������ã�������ÿ��ͨ���ò��Ƽ������Ԫ�ص�����Ϊ g
��ͼ��ʾ����0.1mol����ͭ��һ������̼��ϼ��ȣ���ַ�Ӧ���Թ��еĿ����ѱ���ȥ����
��1�������ʵ�����������ɶ��ٿ˶�����̼��������ݻ�ѧ����ʽ��ʽ���㣩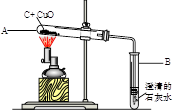
��2��ʵ��ǰ��ֱ������װ��A��װ��B�������������ʾ�������ݢ�ȷ����ش�
a��Ӧ��A�й���ijɷ���_ ��
b���ݢ���ڵ�������_ �����ܵ�ԭ���� ��
| | ��Ӧǰ������g�� | ��Ӧ��������g�� | |
| �� | װ��A | 125.3 | 124.2 |
| �� | װ��B | 152 | 152.3 |
����Cu����һ�ֽ�����������Mg��Fe��Zn�е�һ�֣��γɵķ�ĩ�������ⶨ����ɣ���������ʵ�飺ȡ�÷�ĩ16g�����ձ�����������������Ϊ14%��ϡ����280.0g��4�μ�����ձ��У���ַ�Ӧ���ʣ��Ĺ����������ݼ�¼���£�
| ʵ����� | 1 | 2 | 3 | 4 |
| ����ϡ����������g | 70.0 | 70.0 | 70.0 | 70.0 |
| ʣ�����������g | 13.6 | 11.2 | 8.8 | 8.4 |
���㣨Ҫ��д��������̣���
��1���÷�ĩ��Cu������������
��2���÷�ĩ����һ�ֽ���Ϊ���ֽ�����
��3����3�μ���ϡ�����ַ�Ӧ��������Һ�����ʵ�����������
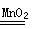 2H2O+O2��)
2H2O+O2��) Ti + 2MgCl2����Ҫ�Ƶ�96 g�ѣ�����������Ҫþ��������
Ti + 2MgCl2����Ҫ�Ƶ�96 g�ѣ�����������Ҫþ��������