��Ŀ����
����Ŀ��ʯ��ʯ����Ҫ���Ʒ֮һ��ijѧУ�о���ѧϰС��Ϊ�˲������ؿ�ʯɽʯ��ʯ��CaCO3������������ȡһЩ��ʯ��Ʒ����ȡϡ����200g��ƽ���ֳ�4�ݣ���4�ν���ʵ�飬������£�
ʵ�� | 1 | 2 | 3 | 4 |
����Ʒ��������g�� | 5 | 10 | 15 | 20 |
����CO2��������g�� | 1.76 | 3.52 | 4.4 | m |
�ʣ���1���ļ���ʵ���п�ʯ��ʣ�ࣿ_____��
��2������m����ֵ��_____��
��3���Լ�������ʯ��ʯ��CaCO3����������_____��
��4���Լ�������ϡ�������ʵ���������_____��
���𰸡���3�͵�4 4.4 80�� 29.2��
��������
��1���ɸ��ݼ���Ʒ������������CO2�ı仯��ϵ�ж��ļ���ʵ���п�ʯ��ʣ�ࣻ
��2����2��3���εõ�CO2�����IJ�ͬ�жϳ���3��ʵ���������Ѿ���Ӧ�꣬��Ʒ�Ѿ�������
��3���ɴӱ�����ѡȡ��Ʒȫ����Ӧ���ʵ�����ݣ�����CaCO3��HCl��Ӧ�Ļ�ѧ����ʽ��CaCO3+2HCl=CaCl2+H2O+CO2![]() ��������CO2�������������Ʒ��CaCO3���������ټ����ʯ��ʯ��CaCO3������������
��������CO2�������������Ʒ��CaCO3���������ټ����ʯ��ʯ��CaCO3������������
��4���ӱ�����Ϣ��֪�����������Է�Ӧ��ȫ������CaCO3��HCl��Ӧ�Ļ�ѧ����ʽ��CaCO3+2HCl=CaCl2+H2O+CO2![]() ���õ���������CO2�����������������HCl���������ټ�������������������
���õ���������CO2�����������������HCl���������ټ�������������������
��1����ʵ��1��2��֪ÿ5g��Ʒ��ȫ��Ӧ�ܵõ�1.76gCO2���壬��3��ʵ���ּ���5g��Ʒֻ��2�������0.88gCO2���壬˵����Ʒ��ʣ�࣬ϡ����㡣���Ե�3�͵�4�η�Ӧ�п�ʯ��ʣ�ࣻ
��2���ɵ�3��ʵ���֪15g��Ʒ�����Ѿ������ˣ�����������Ʒ��Ӧ�����У�����m����4.4g��
��3�����1��ʵ������Ʒ�е�̼��Ƶ�����Ϊx��
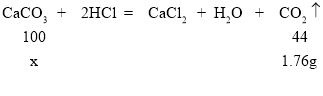
![]()
x=4g
��ʯ��ʯ��Ʒ�Ĵ���Ϊ��![]() ��100%=80%
��100%=80%
��4�����3��ʵ��������HCl������Ϊx��
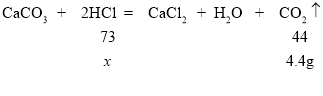
![]()
x=7.3g
��ϡ�������ʵ���������Ϊ![]() ��100%=29.2%
��100%=29.2%

 ��1����Ԫ�¿�������ĩϵ�д�
��1����Ԫ�¿�������ĩϵ�д�����Ŀ������ʵ����Ʋ��ܴﵽʵ��Ŀ�ĵ���
ѡ�� | A | B | C | D |
ʵ����� |
|
|
|
|
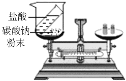
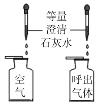
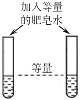
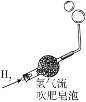
ʵ��Ŀ�� | ��֤�����غ㶨�� | �ȽϺ�������Ϳ����ж�����̼�ĺ��� | ����Ӳˮ����ˮ | ��֤�����ܶȱȿ���С |
A.AB.BC.CD.D
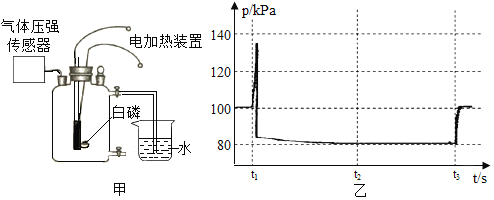
����Ŀ��ij��ȤС���KClO3�ֽⷴӦ�Ĵ��������о�������ͬ�ļ��������£�����ͼװ����ɱ���ʵ��:
��� | KClO3����/g | ���� | ��������/g | �ռ�50mLO2����ʱ��/s |
ʵ��1 | 5 | - | - | 171 |
ʵ��2 | 5 | MnO2 | 0.5 | 49 |
ʵ��3 | 5 | Fe2O3 | 0.5 | 58 |
ʵ��4 | 5 | KCl | 0.5 | 154 |
��1������ʵ��1��Ŀ����_____��
��2����������3�ִ����Ĵ�Ч����ѵ���_____��
��3��д��ʵ��3��KClO3�ֽ�Ļ�ѧ����ʽ:_____��
��4����ʵ��1��ʵ��4��֪��KCl_____�������������������������á�ά�ּ����������䣬��ʵ��1�ټ����ռ��ռ�50mLO2������ʱ����������171s������ԭ��_____��
��5��Ҫ�Ƚ�KClO3�ֽⷴӦ�в�ͬ�����Ĵ�Ч�������˲����ռ�50mLO2����ʱ���⣬�����Բ�����ͬʱ����_____��
��6����ͼװ�ò���һ����������غͶ������̼��ȷֽ�����O2�������ʵ��ʱӦ_____��ѡ����������������������������������ʱ��ʼ�ռ����塣

���������ø�װ�ò����ռ���������������������ļ������裺
A������Ͳ����Һ��ʹ֮��ƽ
Bʹ�Թܺ���Ͳ�ڵ����嶼��ȴ������
C��ȡ��Ͳ����������
��������������ȷ˳���ǣ�_____������д������ţ���
����ʵ�����ʱ����Ͳ�ڵ�Һ�����ˮ���е�Һ�棬Ҫʹ��Ͳ����Һ��ĸ߶���ͬ�ɲ��õIJ�����_____���������������
��ijͬѧ��ʵ����������غͶ�������������������һ��ʱ���ֹͣʵ�飨�����δ��ȫ�ֽ⣩�������ʣ�����������ֽ���Ԫ�ص����������ֱ�Ϊ����Ԫ����������11.0%����Ԫ�ص���������39.0%������ʣ����������Ԫ�ص���������Ϊ_____��
A��10.5% B��14.5% C��25.5% D��50%