��Ŀ����
����Ŀ��ij��ѧ��ȤС��Ϊ����֤�����غ㶨�ɣ�����þ���ڿ�����ȼ�յ�ʵ�顣
��1��С������ȼ�ղ�����������ڷ�Ӧ��þ������������Ϊ�����Ӧ�����������غ㶨�ɣ���_____���ͬ�⡱��ͬ�⡱��С���Ĺ۵㣬��Ϊ_____��
��2��С�찴��ͼװ�øĽ�ʵ�飬��֤�������غ㶨�ɣ�ȴ���ֲ����л���������ɫ���塣
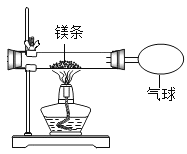
��������⣩��ɫ������ʲô�أ�
���������ϣ�������þΪ��ɫ���壻
��þ���뵪�����ҷ�Ӧ���ɻ�ɫ�ĵ���þ��Mg3N2�����壬д��������þ��Ӧ�Ļ�ѧ����ʽ_____��
�۵���þ����ˮ���ҷ�Ӧ������������������ʹʪ��ĺ�ɫʯ����ֽ������
��ʵ�������
�������� | ʵ������ | ʵ����� |
_____ | _____ | ��ɫ����ΪMg3N2 |
���������룩��ɫ������Mg3N2
��ʵ��̽���������ʵ�飬��֤����
����˼�뽻����������N2�ĺ���Զ����O2�ĺ�������þ���ڿ�����ȼ�����ɵ�MgOȴԶ����Mg3N2��Ϊʲô�أ�����������Ľ���_____��
���𰸡���ͬ�� ��Ϊþȼ����þ������������ȵķ�Ӧ�������������������� 3Mg+N2![]() Mg3N2 ȡ������ɫ�������Թ��У�����������ˮ������ʪ��ĺ�ɫʯ����ֽ�����Թ� �Թ��������������ʪ��ĺ�ɫʯ����ֽ���� O2��N2��ѧ���ʻ��ã��������÷֣�
Mg3N2 ȡ������ɫ�������Թ��У�����������ˮ������ʪ��ĺ�ɫʯ����ֽ�����Թ� �Թ��������������ʪ��ĺ�ɫʯ����ֽ���� O2��N2��ѧ���ʻ��ã��������÷֣�
��������
��1��С������ȼ�ղ�����������ڷ�Ӧ��þ������������Ϊ�����Ӧ�����������غ㶨�ɣ��Ҳ�ͬ�⣬��Ϊ�κλ�ѧ��Ӧ����ѭ�����غ㶨�ɣ�þȼ����þ������������ȵķ�Ӧ����������������������
��2���ڵ�����þ��Ӧ���ɵ���þ����ѧ����ʽΪ3Mg+N2![]() Mg3N2��
Mg3N2��
[ʵ�����]
���������Ϣ����֤��ɫ�����Ƿ��ǵ���þ��ֻ��Ҫ��ˮ����֤�Ƿ��а����������ɡ��������裺ȡ������ɫ�������Թ��У�����������ˮ������ʪ��ĺ�ɫʯ����ֽ�����Թܣ�
ʵ�������Թ��������������ʪ��ĺ�ɫʯ����ֽ������
[��˼�뽻��]
������N2�ĺ���Զ����O2�ĺ�������þ���ڿ�����ȼ�����ɵ�MgOȴԶ����Mg3N2������������O2��N2��ѧ���ʻ��á�

����Ŀ��ijͬѧ���⻯��(CaH2)���Ʊ������ʽ���������̽����
���Ķ����ϣ�
��H2��Ƽ��ȿ��Ƶ�CaH2��
�ڸƺ��⻯�ƶ��ܺ�ˮ��Ӧ�������ﶼ��һ�ּ��һ�����塣
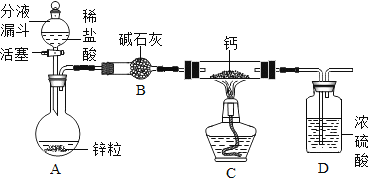
��ʵ��̽������Ƶ���ȡװ����ͼ��ʾ��
(1)��ʯ�ҵ���Ҫ�ɷ�Ϊ�����ƺ��������ƣ�װ��B��������_______������������ʵ��װ�ý���ʵ�飬ʵ�鲽�����£����װ�������ԡ�װ��ҩƷ����Һ©���������˺��ʵ�鲽����ȷ��˳����____(�����)��
A ���ȷ�Ӧһ��ʱ��
B �ռ�װ�����Ҷ˵��ܿڴ������岢�����䴿��
C �رշ�Һ©������
D ֹͣ���ȣ������ȴ
(2)�Ʊ�CaH2ʵ�������ȡ������Ӧ�����С�ļ���ˮ�У��۲쵽�����ݲ���������Һ�е����̪��Һ����______ɫ����ͬѧ�ݴ��жϣ�ʵ����ȷ���⻯�����ɣ�����ͬѧ��Ϊ���Ľ��۲�һ����ȷ��ԭ����_______��
(3)ȡ���������Ƶõ�CaH2��Ʒ�ӵ�������̼������Һ�У������������ݣ����˵���������Һ�������������ijɷ���̼��ƣ���ȼ���������壬����ʵ���ɫ�����������ȼ�ղ���ͨ�����ʯ��ˮ�У��������������Ϊ______(д��ѧʽ)��
(4)����Һ�����ʵijɷ��������²²Ⲣ����ʵ�飺
����һ��NaOH �������NaOH��Ca(OH)2
��������NaOH��Na2CO3 �����ģ�NaOH��Na2CO3��Ca(OH)2
�������ۣ����һ����Ϊ�����IJ����������û�ѧ����ʽ˵��ԭ��_________��
��ʵ����֤��
ʵ�� | ���� | ���� |
ʵ��һ��ȡ��Һ�������е�������Na2CO3��Һ | _____ | ����������� |
ʵ�������ȡ��Һ�������м�������ϡ���� | _____ | ���������� |
������������
CaH2������ұ��ҵ����������Ϊ��������������������126�˴�����CaH2����������ˮ�У������Ͽɻ������____����(���³�ѹ�£��������ܶ�Ϊ0.09g/L���������һλС��)
����Ŀ��ij��ѧС��̽�������ķ�Ӧԭ����
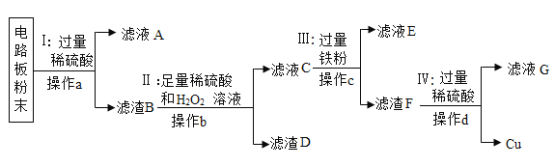
��һ������ͼװ�ûش����⣨������������Ʒ�е�Fe2O3��ȫ��Ӧ�������������ʲ����뷴Ӧ��:
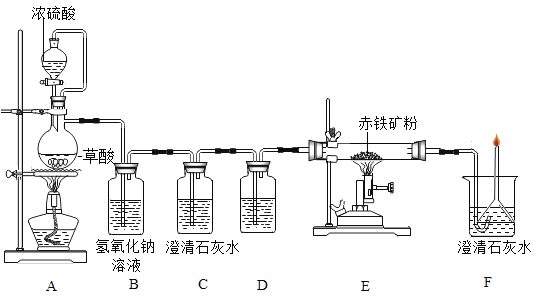
��1��Aװ�õ���������ȡһ����̼����Ӧ�Ļ�ѧ����ʽΪ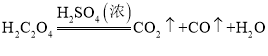 �����ݷ�Ӧװ���жϣ���ѧ����ʽ�л�ȱ�ٵ�������___________________��
�����ݷ�Ӧװ���жϣ���ѧ����ʽ�л�ȱ�ٵ�������___________________��
��2��Cװ�õ�������______��Dװ����ҩƷ��������________��Eװ���з�����Ӧ�Ļ�ѧ����ʽΪ________��F����β��ȼ�յ���Ŀ����______��
��3��ʵ���м�¼���������±����ݴ˼��������������������������Ϊ________��
���������� | �����ܺ�ҩƷ��Ӧǰ���� | �����ܺ�ҩƷ��Ӧ������ |
65.6g | 75.6g | 73.2g |
��4����ͬѧ������Ը���Fװ�������ӵ����������������������������������ͬ��˿�����
˵����Ĺ۵��������_______��
����������ͼװ�ý��мס��ҶԱ�ʵ�飬̽���¶ȶ�CO��Fe2O3��Ӧ��Ӱ�죨�̶�װ�ú�β������װ���ԣ����ù���ҩƷΪ�������
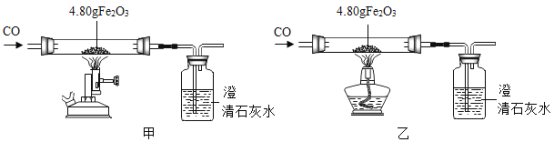
��1����Ӧ�����й۲쵽���ס�������ij���ʯ��ˮ������ǣ�����ƿ�з�����Ӧ�Ļ�ѧ����ʽΪ________��
��2����ȫ��Ӧ�ס���������������Ϊ��ɫ��ĩ���ֱ���������������������ʵ��:
���� | ���� | �������� | �������� |
1 | ������ɫ��ĩ����/g | m1 | m2 |
2 | ȡ��ɫ��ĩ���ô������� | ȫ�������� | ȫ�������� |
3 | ȡ��ɫ��ĩ����������CuSO4��Һ | ��ɫ��ĩ�ܽ⣬�к�ɫ�������� | ���������� |
��ע�������������У�ֻ��Fe3O4�ܱ�����������
�ټ���ĺ�ɫ��ĩ��CuSO4��Һ��Ӧ�Ļ�ѧ����ʽ��_____��
��С��ͬѧ�������Ϻ���Fe3O4�ڳ����²���CuSO4��Һ��Ӧ���ʲ��������������ΪFe3O4��
С�������ͬ��������������ɫ��ĩ�г�����Fe3O4�������ܺ����������ۣ����۵ĺ����ͣ�������ͭ��Һ��ӦҲ��������������
�˽�һ��ȷ������������ɷ֣�С��ͬѧ��������m2ǡ��Ϊ______________g����С���IJ�����ȷ�����m2_____________________������ڡ���С�ڡ������������������������л������������ۡ�