��Ŀ����
����Ŀ���Ķ�������ն���
ij�����������ܲ�Ҷ���������������£�
ԭ�ϡ���ѡ���������ϴ����ˮ�����բ�����բ�����Ρ����ơ���ζ����հ�װ��ɱ������ȴ����Ʒ��
�ܲ�Ҷ�����ƹ���������ķ����̣������������ﵰ��ø����ø�����£������һЩ�����ᡢ����ȷ�ζ���ʡ����ƹ������������������л�������ܲ�Ҷ�ĵ����ʣ��Ӷ�ʹ�����ʺ����½������ơ���ζ�����Ʒ�е����ʺ������ߣ��䵰������Ҫ��Դ�ڽ��ƺ͵�ζ���̼���Ľ��ͼ�ζ���������к��е����ʣ�ζ�����Ȱ������Σ�ʹ��Ʒ�ܲ�Ҷ�����еĵ��������ߡ�
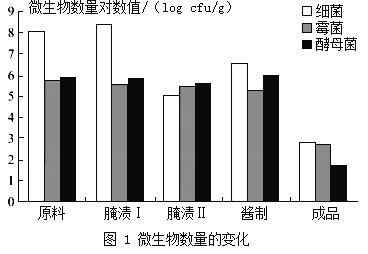
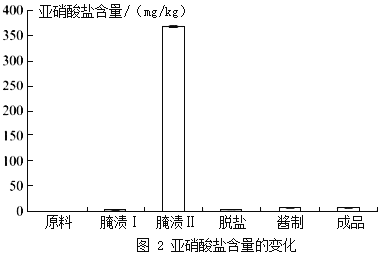
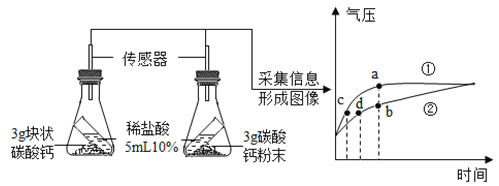
ѡȡԭ�ϡ����բ����բ��ƺͳ�Ʒ����������Ʒ������ϸ����ù���ͽ�ĸ���IJⶨ����ʵ������ͼ1��ʾ�����բ����Ҫ�����������Ϊ����������������ڶ����ڣ�Ѹ�����������բ����Ҫ�����ƹ����е��ηֲ����ĸ���ѹ������������������������Ʋ��������������
�����к�����������ܹ����ڳ�θ���ܣ������ڶ�ͯ�������������������⣬�����е����ʡ�ά����C�����ձ�ϸߣ����к��е�����ƻ��ܴٽ���ͯ�ijɳ�������һЩ�߲˱������ơ��������ϸߣ��������ƺ�õ�Ũ�����ơ���������Ϊ�ḻ����ˣ�����ʳ�ý��˶����彡�����档
���ǣ���������������Ҳ������ϸߵ��������Ρ�����������θ�ᷴӦ�����������ᣨHNO2�����Ȼ��������ȶ��������Ķ�����������ѪҺ��Ѫ�쵰��ϣ������ж������������������������κ����仯��ͼ2��ʾ��
�����������ݻش��������⡣
��1���ơ��������ϸߵ��߲ˣ��������Ƴ�Ϊ���ˣ��ơ����������Ϊ�ḻ������ġ��ơ�������ָ_____������ӡ���ԭ�ӡ���Ԫ�ء�����
��2�����ơ���ζ��Ľ�����Ʒ�е����ʺ������ߣ���Ҫ��Դ��_____��
��3���������ƣ�NaNO2���dz������������Σ�����������θ�ᷴӦ�Ļ�ѧ����ʽΪ_____��
��4������˵����ȷ����_____��
A �ܲ�Ҷ�ķ��������������仯
B �������������У��������κ����ڷ��ͺ��ڴﵽһ����ֵ����Ѹ���½�
C �������������У�ù���ͽ�ĸ��������������½������ƣ�ϸ�������������������
D ù���ͽ�ĸ�����������ơ����ƹ����в�����С
��5������˵��ͯ���ʺϳԽ��ˣ���дһд��Ĺ۵㣬��˵�����ɣ�_____
���𰸡�Ԫ�� ���ƺ͵�ζ���̼���Ľ��ͼ�ζ�� NaNO2+HCl=HNO2+NaCl BD ��ͯ��������ʳ�ý��ˣ���Ϊ�����к�����������ܹ����ڳ�θ���ܣ������ڶ�ͯ�����������к��е�����ƻ��ܴٽ���ͯ�ijɳ��������𰸺������ɣ�
��������
��1������������ɵ���������������ġ��ơ�������ָԪ�أ�
��2�����ơ���ζ�����Ʒ�е����ʺ������ߣ��䵰������Ҫ��Դ�ڽ��ƺ͵�ζ���̼���Ľ��ͼ�ζ���������к��е����ʣ�ζ�����Ȱ������Σ�ʹ��Ʒ�ܲ�Ҷ�����еĵ��������ߣ�
��3���������ƣ�NaNO2���dz������������Σ�����������θ�ᣨ�����ᣩ��Ӧ�����������ᣨHNO2�����Ȼ��ơ�����������θ�ᷴӦ�Ļ�ѧ����ʽΪNaNO2+HCl�THNO2+NaCl��
��4��A���ܲ�Ҷ�ķ��������������µ����ʣ����ڻ�ѧ�仯����ѡ��˵������
B������ͼ2��Ϣ���������������У��������κ����ڷ��ͺ��ڴﵽһ����ֵ����Ѹ���½�����ѡ��˵����ȷ��
C���������������У�ù���ͽ�ĸ���������仯����ֻ�����բ�ͽ���ϸ���������½����ƣ���ѡ��˵������
D��ù���ͽ�ĸ�����������ơ����ƹ����в�����С����ѡ��˵����ȷ��
��5����ͯ��������ʳ�ý��ˡ����ɣ������к�����������ܹ����ڳ�θ���ܣ������ڶ�ͯ�����������к��е�����ƻ��ܴٽ���ͯ�ijɳ������ȡ�

����Ŀ��ij��ѧ��ȤС���ͬѧ������������̼��ʵ������ȡ��������ʵ��ʱ�����ֳ�ʱ�������ʯ��ˮ��ͨ�������̼��ʯ��ˮ�ȱ���ǣ����ֱ���壬���ȳ����Һ���ֱ���ǡ������ʦ��֪�������ܵ�̼������������̼��ˮ��Ӧ���������ܽ��̼����ƣ�CaCO3+CO2+H2O=Ca(HCO3)2��̼����������ֽ⣺Ca(HCO3)2 ![]() CaCO3��+H2O+CO2�������ǶԳ�ʱ�������Һ��ͨ��CO2��Ӧ����Һ�е�������ɲ�����Ũ�����Ȥ��
CaCO3��+H2O+CO2�������ǶԳ�ʱ�������Һ��ͨ��CO2��Ӧ����Һ�е�������ɲ�����Ũ�����Ȥ��
��������⣩
һ����CO2��NaOH��Һ��Ӧ�����Һ�����������ʲô?
���������ϣ�
��1��ͨ������CO2����Ӧ�Ļ�ѧ����ʽΪ�� __________________��
��2��ͨ�����CO2����Ӧ�Ļ�ѧ����ʽΪ��Na2CO3+CO2+H2O=2NaHCO3��
��3��̼�����ζ��ǿ�����ˮ�ģ�BaCO3������ˮ��
��������룩
��1������ΪNa2CO3��
��2������ΪNaHCO3��
��3������ΪNaOH��Na2CO3��
��4������Ϊ___________���ѧʽ����
�����ʵ�飩
ʵ�鲽�� | ʵ������ | ʵ����� |
A. ȡ��Ӧ����Һ�������Թ��У������еμӹ�����BaCl2��Һ | ��____���� | ���루2�������� |
B. ȡ����a�е��ϲ���Һ������ϡ���� | ������ð�� | ���루1���ͣ�3�������� |
���ó����ۣ�
���루4��������
�����۽�����
��д��NaHCO3���ȷֽ�Ļ�ѧ����ʽ�� ___________��
����Ŀ�����A��D����ѡ���������������𣬰�ǰ�������֡���ͼ��ʾ������֧�Թ��н���ʵ�飬��ȫʵ�鷽����
��� | Ŀ�� | ��������� |
A | ����NaCl��Һ��Na2CO3��Һ | ���Թ�1��2�зֱ����������Һ����������֧�Թ��м���_______�� |
B | _______ | ���Թ�1�м���һ�����5mLˮ�����Թ�2�м���һ�����5mL���͡��۲쵽1�й��弸�����ܽ⣬2�й���ȫ���ܽ⡣ |
C | �Ƚ�п������ͭ�Ľ������ | ���Թ�1�м���ZnSO4��Һ���Թ�2�м���CuSO4��Һ�� ���ٷֱ�����֧�Թ��в�����˿���۲쵽��������_______�� |
D | ________ | ���Թ�1��2�о�����һС�����80����ˮ��û���� ���õ��ܶ��Թ�2�а���ͨ�������� |
����Ŀ����ѧ��ȤС���ͬѧ��þ��������ˮ����У�����ȡ��������Һ���μӷ�̪��Һ����Һ��Ϊ��ɫ�������ڿ�����һ��ʱ�������Һ�ĺ�ɫ��ȥ��Ϊ��Ū�����ɫ��ȥ��ԭ�����������̽���:��������:þ������ˮ��Ӧ����������������þ���÷�Ӧ�Ļ�ѧ����ʽΪ_____��
��λͬѧ�ֱ�Ժ�ɫ��ȥ��ԭ���������²���:
��:������ʵ�����õķ�̪��Һ������ɵģ�
��:��Һ����������е����ʷ����˷�Ӧ��
��:������þ���ܽ�����¶ȵĽ��Ͷ���С��
(1)ͬѧ�Ǹ���ʵ��������Ϊ�ײ��벻��ȷ��������____��
(2)ͬѧ�������ʵ������֤�Ҳ��룬ʵ���¼���±���
ʵ�鷽�� | ʵ������ |
��������ɫ��Һ�ڿ����к��·���һ��ʱ�� | ��ɫ����ȥ |
��������ɫ��Һ�ڸ������������º��·���һ��ʱ�� | ��ɫ����ȥ |
�ɸ�ʵ���֪�Ҳ���______(������ȷ����������ȷ��)
(3)������������ȷ�ģ������ʵ��֤��������±���
ʵ�鷽�� | ʵ������ |
��������ɫ��Һ���·���һ��ʱ�� | ��ɫ����ȥ |
____ | _____ |
(4)��˼:
�������Ϊʲô�ڼ��ォ����ˮ�տ�������ˮ���У���ʼ���������һ��ʱ�����ڱ������ɫ������_____��
������۽ǶȽ���Ϊʲô����ˮ��������þ����ʹ��̪��������ˮ�в�����_____��