��Ŀ����
�ձ���װ��һ�������������ͭ�Ļ����Һ����֪����Һ�к�H2SO4������Ϊ9��8g��ijͬѧΪ�ⶨ�û����Һ������ͭ�����������ձ�������10%��NaOH��Һ���õ�������������¼���£�
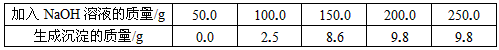
��1���õ�������������Ϊ g���û����Һ������ͭ������Ϊ g��
��2���μӷ�Ӧ��NaOH��Һ���������Ƕ��ٿˣ���Ҫ��д��������̣�
��3�������ڸû����Һ�м���NaOH��Һ���������ɳ��������仯��ϵ�����ߡ�

��1���õ�������������Ϊ g���û����Һ������ͭ������Ϊ g��
��2���μӷ�Ӧ��NaOH��Һ���������Ƕ��ٿˣ���Ҫ��д��������̣�
��3�������ڸû����Һ�м���NaOH��Һ���������ɳ��������仯��ϵ�����ߡ�
��1��9��8 16��0 ��2��160
��3��
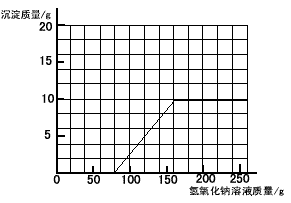
��3��

�����������1�����ϱ���¼�����ݿ�֪���տ�ʼ����NaOH��Һ����û�����ɳ�����ԭ����ԭ�ձ���װ��һ�������������ͭ�Ļ����Һ�������NaOH��Һ���Ⱥ����ᷴӦ��H2SO4 + 2NaOH=Na2SO4 + 2H2O��Ȼ������NaOH��Һ�IJ��ϼ��룬������NaOH��Һ������ͭ��Ӧ���ɳ�����2NaOH + CuSO4=Cu��OH��2�� + Na2SO4
����������NaOH��Һ�ļ������룬���ɵij�������Ҳ��Խ��Խ�����ձ���9��8g���䣬��ʾ��Ӧ����������ͭ�������꣬��������ͭ�ͳ���Cu��OH��2��������ϵ�������������ͭ������
��2��ͬ������ѧ��Ӧ��2NaOH + CuSO4=Cu��OH��2�� + Na2SO4�У�����NaOH�����Cu��OH��2��������ϵ��Ҳ������μӷ�Ӧ��NaOH���ʵ���������Ŀ��֪NaOH��Һ���������������������Ϳ�������μӷ�Ӧ��NaOH��Һ��������
��3����ͼ��һ��Ҫע�������㣺��ʼ�㣨��ʾij��Ӧ�Ŀ�ʼ����ת�۵㣨��ʾij��Ӧ�Ľ�����������ղŷ����ģ��տ�ʼ����NaOH��Һ����û�����ɳ�����ԭ����ԭ�ձ���װ��һ�������������ͭ�Ļ����Һ�������NaOH��Һ���Ⱥ����ᷴӦ��H2SO4 + 2NaOH=Na2SO4 + 2H2O����ԭ��Һ�к�H2SO4������Ϊ9��8g�����ݻ�ѧ����ʽ��NaOH��H2SO4��������ϵ���������Ҫ��9��8g H2SO4��ȫ��Ӧ����Ҫ��NaOH��������Ҳ����ͼ���е���ʼ�㣬����Ӧ����ʱ���ĵ�NaOH�������ڵڣ�2�����Ѿ��������ͼ���е�ת�۵㣬��ʱ��Һ������ͭҲ��ȫ�����ģ����Գ�������Ҳ�Ͳ��ٷ����仯
��2���⣺�������ᷴӦ��NaOH������Ϊx ����CuSO4��Ӧ��NaOH������Ϊy��
H2SO4+ 2NaOH��Na2SO4 + 2H2O �� CuSO4 + 2NaOH ��Cu��OH��2��+ Na2SO4
98 80 �� 80 �� 98
9��8g x �� y �� 9��8g
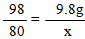
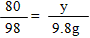
x = 8g y =8g
�ʲμӷ�Ӧ��NaOH��Һ��������Ϊ
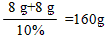
�����������вμӷ�Ӧ��NaOH��Һ����������160�ˡ�

��ϰ��ϵ�д�
 ���⿼����Ԫ���Ծ�ϵ�д�
���⿼����Ԫ���Ծ�ϵ�д� ��У���˳�̾�ϵ�д�
��У���˳�̾�ϵ�д� ��У���һ��ͨϵ�д�
��У���һ��ͨϵ�д�
�����Ŀ


