��Ŀ����
����Ŀ��I.ij��ѧ��ȤС���������ϵ��ʵ�顣�������ͼ�ش����⣺
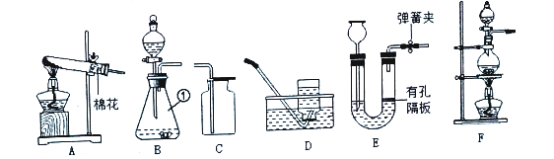
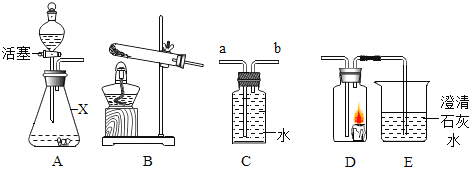
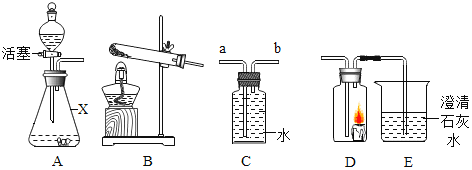
(1)ͼB�������ٵ�������______��ʵ������Aװ����ȡ����ʱ�����Թܿڷ�һ������Ŀ����_____��
(2)ʵ������˫��ˮ��ȡ�����Ļ�ѧ��Ӧ����ʽΪ________����ʵ������ȡCO2ʱ�ܿ���ʹ��Ӧ��ʱ������ֹͣ��Ӧ��ѡ�õķ�������װ����_______������ţ�
(3)ʵ���ҳ������Ȼ����Һ����������(NaNO2)��Һ��ϼ�����ȡ������Ӧѡ�õ���ȡװ����________.(�����)��
IIʵ������һƿֻ������ˮ��̼�������Ʒ��Ϊ�ⶨ����̼����淋�������������ѧ��ȤС����������ͼ��ʾװ�ý���ʵ��(��װ�����������ã�װ��B��C����װҩƷ����������ʯ��ʯ�����ƺ��������ƵĻ����)����֪̼��������ȷֽ�Ļ�ѧ����ʽΪ��NH3HCO3![]() NH3��+H2O��+CO2����NH3�ܱ�����Һ��ȫ���ա�
NH3��+H2O��+CO2����NH3�ܱ�����Һ��ȫ���ա�
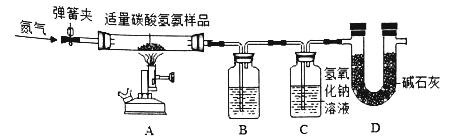
����ʵ�鲽������
�ٴ��ɼУ�ͨ��һ��ʱ��ĵ���������B��Cװ�õ�������
�ڹرյ��ɼУ���̼�������Ʒ���ȣ�
�۵���Ʒ��Ӧ��ȫ��ֹͣ���ȣ���.....��ֱ����������ȴ���ٴβ���B��Cװ�õ�������
��ʵ����ϣ����װ��B��C��ҩƷ�������ֱ�������3.6�ˡ�4.4�ˣ�
��ش��������⣺
(1)�������ͨ�뵪����Ŀ����__��װ��B��ʢ�ŵ�Һ����_____����������____��
(2)װ��C�з�����Ӧ�Ļ�ѧ����ʽΪ____��
(3)������У�ֹͣ���Ⱥ�Ӧ���еIJ�����___�����ȱ��Dװ�ã���õ�̼����淋�����������_______(�ƫ��ƫС�����䡱����ȷ����)��
(4)����Ʒ��̼����淋���������Ϊ______��
���𰸡���ƿ ��ֹ����ʱ������ط�ĩ���뵼�� 2H2O2![]() 2H2O+O2�� E A �ž�װ���ڵĿ��� Ũ���� ����ˮ�����Ͱ��� 2NaOH+CO2==Na2CO3+H2O ���ɼУ�ͨ��һ��ʱ��ĵ��� ƫ�� 98.75%
2H2O+O2�� E A �ž�װ���ڵĿ��� Ũ���� ����ˮ�����Ͱ��� 2NaOH+CO2==Na2CO3+H2O ���ɼУ�ͨ��һ��ʱ��ĵ��� ƫ�� 98.75%
��������
��
��1��ͼB�������ٵ���������ƿ��������ƿ��ʵ������Aװ����ȡ����ʱ�����Թܿڷ�һ������˵�����ø��������������������Ŀ���Ƿ�ֹ����ʱ������ط�ĩ���뵼�ܣ������ֹ����ʱ������ط�ĩ���뵼�ܣ�
��2��ʵ�����÷ֽ�˫��ˮ��ȡ������ͬʱ����ˮ����ѧ��Ӧ����ʽ����2H2O2![]() 2H2O+O2������ʵ������ȡCO2ʱ�ܿ���ʹ��Ӧ��ʱ������ֹͣ��Ӧ��ѡ�õķ�������װ����E�����ö�����ֹˮ����ϣ�����Ӧ��Ҫֹͣʱ���н�ֹˮ�У�ʹ���ɵĶ�����̼���������γ�ѹǿ�������Һ����룬ʹ��Ӧֹͣ������E��
2H2O+O2������ʵ������ȡCO2ʱ�ܿ���ʹ��Ӧ��ʱ������ֹͣ��Ӧ��ѡ�õķ�������װ����E�����ö�����ֹˮ����ϣ�����Ӧ��Ҫֹͣʱ���н�ֹˮ�У�ʹ���ɵĶ�����̼���������γ�ѹǿ�������Һ����룬ʹ��Ӧֹͣ������E��
��3��ʵ���ҳ������Ȼ����Һ����������(NaNO2)��Һ��ϼ�����ȡ��������Ӧ����Һ�壬��Ӧ������Ҫ���ȣ�Ӧѡ�õ���ȡװ����F������F��
��
��1�������еĶ�����̼��ˮ�����ᱻװ���е�����������Һ��Ũ�������գ���������ͨ�뵪����Ŀ�����ž�װ���ڵĿ����������ž�װ���ڵĿ�����Ũ���������ˮ�ԣ��������հ���������װ��B��ʢ�ŵ�Һ����Ũ���ᣬ����Ũ���ᣬ������������ˮ�����Ͱ�������������ˮ�����Ͱ�����
��2��װ��C�з��������������ƺͶ�����̼��Ӧ����̼���ƺ�ˮ����Ӧ�Ļ�ѧ����ʽ����2NaOH+CO2=Na2CO3+H2O��
��3��������У�ֹͣ���Ⱥ�Ӧ���ɼм���ͨ����һ��ʱ�䣬ʹ���ɵĶ�����̼��������ˮ������ַ�Ӧ�����еIJ���������ɼУ�ͨ��һ��ʱ��ĵ��������ȱ��Dװ�ã������еĶ�����̼����������Ʒ�Ӧ��ʹ����ƫ��õ�̼����淋�����������ƫ����ƫ��
��4��ʵ����ϣ����װ��B��C��ҩƷ�������ֱ�������3.6�ˡ�4.4�ˣ�˵����Ʒ����=3.6g+4.4g=8.0g��װ��C�����ӵ��������������������յĶ�����̼����������֪��Ӧ���ɶ�����̼����Ϊ4.4g�����ݻ�ѧ����ʽ���м��㣬����Ʒ�к���̼����淋�����Ϊx��
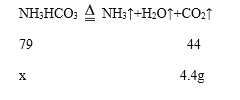
���x=7.9g������Ʒ��̼����淋���������=![]() ��100%=98.75%������98.75%��
��100%=98.75%������98.75%��

 ��У����ϵ�д�
��У����ϵ�д�����Ŀ������ѧ��ʵ��̽���봴����ʶ�ǻ�ѧѧ����������֮һ��ijУ��ѧ��ȤС���ͬѧ����ʦ��ָ���£���ij���Ͻ��ĩ������ͭ�Ĵ������������̽����
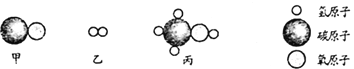
���������ϣ�����������������Һ������Ӧ������ʽΪ![]()
������![]() ����ˮ����
����ˮ����![]() ��
��![]() ����������������Һ��Ӧ��
����������������Һ��Ӧ��
�����룩����1���úϽ��ĩ�г����⣬��������
����2���úϽ��ĩ�г����⣬������__________�������ƣ�
����3���úϽ��ĩ�г����⣬����������ͭ
��ʵ��̽��������ʵ�����ѡ����Լ���ϡ���ᡢ![]() ��Һ
��Һ
ʵ�鷽�� | ʵ������ | ���� |
��ȡһ�����ĺϽ��ĩ���ӹ�����__________����ַ�Ӧ����ˣ��������á� | ��ĩ�����ܽ⣬�������ݲ����� | �úϽ���һ������__________�� |
��ȡ����������������ӹ�����__________����ַ�Ӧ | ���������ܽ⣬�������ݲ�������Һ����ɫ��Ϊdz��ɫ�� | �úϽ���һ������__________�� |
��̽�����ۣ�__________��
����˼��һ����˵�����ý�������������ᷴӦ���������ᡢ��ܷ�Ӧ��˵����������������ʡ����ڿ�����Ҳ������ʴ�����ʣ�д�����ڿ�������ʴԭ��Ļ�ѧ��Ӧ����ʽ__________��