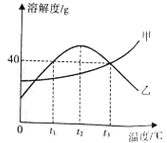
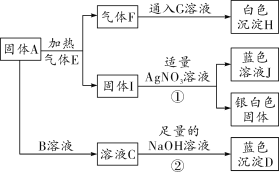
��Ŀ����
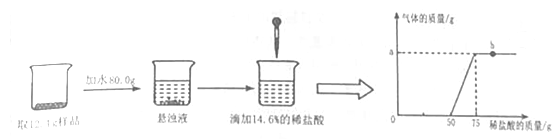
����Ŀ����2016������Է��ģ�⣩�ձ���װ��һ���������������ͭ�Ļ����Һ����֪����Һ�к���HCl������Ϊ3.65 g��ijͬѧΪ�ⶨ�û����Һ������ͭ�����������ձ�������10%��NaOH��Һ���õ��������������±���ʾ��
����NaOH��Һ������/g | 20.0 | 40.0 | 60.0 | 80.0 | 100.0 |
���ɳ���������/g | 0.0 | 0.00 | 2.45 | 4.90 | 4.90 |
��ش��������⣺
��1�����յõ�������������Ϊ����g��
��2���μӷ�Ӧ��NaOH��Һ�����������������С�������һλ����
���𰸡���1��4.90��2��80.0 g
��������
��2���⣺��NaOH��HCl��Ӧ������Ϊx��������ͭ��Ӧ������Ϊy
NaOH��HCl=== NaCl��H2O
40 36.5
x 3.65 g
![]() ��
��![]() ����x��
����x��![]() ��4.0 g
��4.0 g
2NaOH��CuSO4=== Cu��OH��2����Na2SO4
80 98
y 4.9 g
![]() ��
��![]() ��y��
��y��![]() ��4.0 g
��4.0 g
�μӷ�Ӧ��NaOH��������Ϊ4.0 g��4.0 g��8.0 g
��NaOH��Һ������Ϊ![]() ��80.0 g
��80.0 g
�𣺲μӷ�Ӧ��NaOH��Һ����������80.0 g��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ��ij��ѧ��ȤС���ȡ4.0 gʯ��ʯ��Ʒ����40 gϡ�����4�μ�����Ʒ�У��������ʲ���ӦҲ���ܽ⣩����ʵ���������£�
ϡ��������� | ʣ���������� |
��һ�μ���10 g | 3.0 g |
�ڶ��μ���10 g | 2.0 g |
�������10 g | 1.0 g |
���Ĵμ���10 g | 0.6 g |
����㣺
��1��4.0 gʯ��ʯ��Ʒ��̼��Ƶ�������_____g��
��2����ϡ�������������������