��Ŀ����
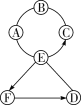
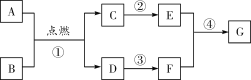
����Ŀ��A��B��C��D��E�������ʾ�Ϊ���л�ѧ�г��������ʣ�����֮��Ĺ�ϵ��ͼ��ʾ���֮������߱�ʾ�ܷ�����ѧ��Ӧ��A��Ŀǰ�������ߵ�һ�ֽ������ʣ�B��C��D�ֱ����ᡢ����е�һ�֣�ũҵ�ϳ���B��D����Һ����ũҩ������Һ��C������ʵ������ȡ������̼��E��һ���Σ�����ˮ��Һ�Լ��ԣ�����������ϴ�Ӽ���

(1)A�Ļ�ѧʽ��________��
(2)A��D��Ӧ�Ļ�ѧ����ʽΪ__________________________��
(3)E���׳���____________��
(4)��ҵ�Ͽ�ͨ��B��E��Ӧ��ȡ�ռ��Ӧ�Ļ�ѧ����ʽΪ___________________��
���𰸡�Fe Fe��CuSO4= Cu��FeSO4 �մ� Na2CO3��Ca(OH)2= CaCO3����2NaOH
��������
![]() ��һ����A���������ߵ�һ�ֽ������ʣ�A��Fe��
��һ����A���������ߵ�һ�ֽ������ʣ�A��Fe��
�ڶ�����B��C��D�ֱ����ᡢ����е�һ�֣�ũҵ�ϳ���B��D����Һ����ũҩ������Һ��C������ʵ������ȡ������̼�����Ƴ�B��D�ֱ���CuSO4��Ca(OH)2��һ�֡�C��HCl��E��һ���Σ�����ˮ��Һ�Լ��ԣ�����������ϴ�Ӽ������Ƴ�EΪNa2CO3��
��������A��C��D�ɷ�Ӧ��B��D��E�ɷ�Ӧ�����Ƴ�B��Ca(OH)2��D��CuSO4��

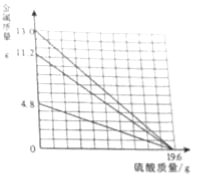
����Ŀ����2016������Է��ģ�⣩�ձ���װ��һ���������������ͭ�Ļ����Һ����֪����Һ�к���HCl������Ϊ3.65 g��ijͬѧΪ�ⶨ�û����Һ������ͭ�����������ձ�������10%��NaOH��Һ���õ��������������±���ʾ��
����NaOH��Һ������/g | 20.0 | 40.0 | 60.0 | 80.0 | 100.0 |
���ɳ���������/g | 0.0 | 0.00 | 2.45 | 4.90 | 4.90 |
��ش��������⣺
��1�����յõ�������������Ϊ����g��
��2���μӷ�Ӧ��NaOH��Һ�����������������С�������һλ����