��Ŀ����
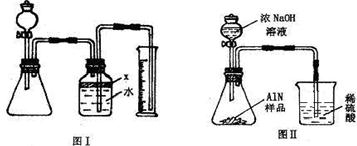
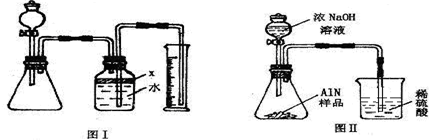
��������AlN����һ�����������ϣ��㷺Ӧ���뼯�ɵ�·��������ij�������к���̼�����������ʣ�����ͼ���е�һЩװ�������м��飬ʹ��������Ʒ��NaOH��Һ��ӦAlN+NaOH+H2O=NaAlO2+NH3�������ݷ�Ӧ�������ɰ�����������ⶨ��Ʒ�еĵ�����������������������ʵ��������ȷ�����ʵijɷ֣�ʵ���е���������Բ��ƣ�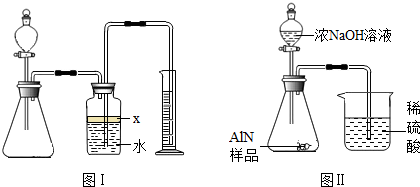
��1��ʵ���йز���Ϊ��a������ƿ�з���������AlN��Ʒ��b���ӷ�Һ©������ƿ�м��������ŨNaOH��c������װ�õ������ԣ�d���ⶨ�ռ���ˮ���������ȷ�IJ���˳��Ϊ
��2���������м��װ�������Եķ�����
��3�����ƿ�е��Լ�X��ѡ��
��4�����ƿ��Һ��û��װ�����Ϸ����������ռ䣩��ʵ����NH3�������
��5��ʵ����������۲쵽��ƿ�л��й��壬����Ʒ�к��е�������
��6����ʵ���в����Ʒ������Ϊwg�����������ΪaL������£�������Ʒ��AlN����������Ϊ
��7�����˸���ͼ��װ�ý���ͬ��ʵ�飬ͨ���ⶨ�ձ��������������ȷ����Ʒ��AlN����������������Ϊ�Ƿ���У�
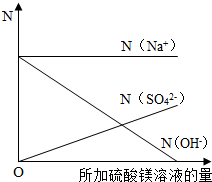
��8����mg 20%������������Һ�еμ�2��3�η�̪��Һ��Ȼ��߽������������м�������þ��Һ������Һ�ĺ�ɫ��ȫ��ȥʱ����ȥ�������Ƶ�ʣ����Һ������Ϊ3mg��
�ټ�����������þ��Һ������������
����N��ʾ��Һ�����ӵ���Ŀ����ͬ�����������ӷ���ע��[��N ��Na'����ʾ�����ӵ���Ŀ]���뽨������ϵ�����������μӹ����и������ӵ���Ŀ����Һ�IJ��ϼ�����仯�Ĺ�ϵͼ��
��������1����ȡ����ʱ��Ϊ��ֹװ��©��Ӧ������װ�ú���������װ�������Լ�飬ȷ��װ�ò�©�������ȼӹ�����Һ���ԭ�����ҩƷ��������������ռ��������
��2���رշ�Һ©���Ļ�����װ�����γɷ�ջ����������װ�ý��м��ȣ�װ�����������������������������ã��ͻ�۲쵽���ƿ���Ҳർ��ˮ������������ʱˮ���������䣻
��3�������İ�����������ˮ��Ϊ��ֹ��������ˮ��Ҫ��������ˮ���룬���Ӧѡ�����백���������õ�Һ����Ϊ����Һ��
��4������ʵ���Ŀ�����ڲⶨ�������������������ռ����������壬��ˣ����ƿ�ڵ�ԭ�����岻�ڲ�����Χ�ڣ�
��5��������������������������������Һ��Ӧ���γ�ƫ��������Һ��ˮ�Ͱ�������ַ�Ӧ���й������ʵIJ�������̼����������������Һ��Ӧ��
��6����Ʒ��AlN����������=
��100%����ˣ���Ҫ���ݷ�Ӧ�Ļ�ѧ����ʽ���ɲ�����������������μӷ�Ӧ��������������
��7�������������ᷴӦ��������臨�ʹϡ������Һ�������ӣ������ڷ�Ӧ��Ϊ���Ҷ���ʹϡ���ᵹ����������ձ���������ȷ��Ϊ�����������֣����ڵ���ĩ�˰�װ©����ֹ������
��8��������������Һ�ʼ��ԣ���ʹ��ɫ��̪��죻��������������þ��Һ��Ӧ������������þ��������������Һ����������Һ�����ԣ���ǡ����ȫ��Ӧʱ������Һ����ʹ��̪��������ɫ�����ݷ�Ӧ�Ļ�ѧ����ʽ�����������Ƶ������ɼ���ǡ����ȫ��Ӧʱ��������þ�����������������غ㶨�ɣ���������þ��Һ������=��Ӧ����Һ����+������þ��������-����������Һ������
����������������þ��Ӧ����������þ�����������ƣ���Һ����������Ŀ���䣬��������þ�ĵμ���������Ӳ������ࡢ���������Ӳ��ϼ��٣�ԭ��Һ����þ�����ҵ�������þ����������������þ���������Եμ�ǰ����Һ��ʼ�ղ���þ���ӣ�
��2���رշ�Һ©���Ļ�����װ�����γɷ�ջ����������װ�ý��м��ȣ�װ�����������������������������ã��ͻ�۲쵽���ƿ���Ҳർ��ˮ������������ʱˮ���������䣻
��3�������İ�����������ˮ��Ϊ��ֹ��������ˮ��Ҫ��������ˮ���룬���Ӧѡ�����백���������õ�Һ����Ϊ����Һ��
��4������ʵ���Ŀ�����ڲⶨ�������������������ռ����������壬��ˣ����ƿ�ڵ�ԭ�����岻�ڲ�����Χ�ڣ�
��5��������������������������������Һ��Ӧ���γ�ƫ��������Һ��ˮ�Ͱ�������ַ�Ӧ���й������ʵIJ�������̼����������������Һ��Ӧ��
��6����Ʒ��AlN����������=
| ��Ʒ��AlN������ |
| ��Ʒ����wg |
��7�������������ᷴӦ��������臨�ʹϡ������Һ�������ӣ������ڷ�Ӧ��Ϊ���Ҷ���ʹϡ���ᵹ����������ձ���������ȷ��Ϊ�����������֣����ڵ���ĩ�˰�װ©����ֹ������
��8��������������Һ�ʼ��ԣ���ʹ��ɫ��̪��죻��������������þ��Һ��Ӧ������������þ��������������Һ����������Һ�����ԣ���ǡ����ȫ��Ӧʱ������Һ����ʹ��̪��������ɫ�����ݷ�Ӧ�Ļ�ѧ����ʽ�����������Ƶ������ɼ���ǡ����ȫ��Ӧʱ��������þ�����������������غ㶨�ɣ���������þ��Һ������=��Ӧ����Һ����+������þ��������-����������Һ������
����������������þ��Ӧ����������þ�����������ƣ���Һ����������Ŀ���䣬��������þ�ĵμ���������Ӳ������ࡢ���������Ӳ��ϼ��٣�ԭ��Һ����þ�����ҵ�������þ����������������þ���������Եμ�ǰ����Һ��ʼ�ղ���þ���ӣ�
����⣺��1��Ӧ�Ƚ���װ�������Լ��飬Ȼ�����μ������ҩƷ��Һ��ҩƷ�������������ų�ˮ�IJ�����ȷ���������������
�ʴ�Ϊ��cabd��
��2��ͨ������װ��������ʹ�������������װ��©����۲쵽װ���������Ա仯��������������ã����ƿ���Ҳർ��ˮ������������ʱˮ�������أ�
�ʴ�Ϊ���رշ�Һ©������������ƿ�����ƿ���Ҳർ��ˮ������������ʱˮ���������䣻
��3���ƾ������ͺ����Ȼ�̼��Ȼ�������백��������Ӧ��������ȴ�����ӷ����ӷ������������ʵ����Ӱ����һӷ�����������백����ˮ�Ӵ������ã��ټ�֮�ƾ�������ˮ���Ȼ�̼�ܶȱ�ˮ�����ܴﵽ�����Ŀ�ģ���ֲ���ͼȲ�����ˮҲ���ӷ������Ѱ�����ˮ���и��룻
�ʴ�Ϊ��C��
��4������ʵ���Ŀ�����ڲⶨ�������������������ռ����������壬��ˣ����ƿ�ڵ�ԭ�����岻�ڲ����ڣ�����Բ����������Ӱ�죻
�ʴ�Ϊ�����䣻
��5������̼����������������Һ������Ӧ��������������������������������Һ�����ܽ����ʧ����ˣ������й������ʱ��˵��ԭ�����к���̼��
�ʴ�Ϊ��̼��
��6���赪����������Ϊx
AlN+NaOH+H2O=NaAlO2+NH3��
41 17
y
��17g
=
y=
g
��Ʒ��AlN����������=
��100%=
%
�ʴ�Ϊ��
%��
��7��������������ϡ��������ֵ�������ˣ���װ�ò���ȷ���������������������ڵ���ĩ������©��������Һ���ϣ��հ�����������ʱ���ձ���Һ���½�������Ӵ������Է�ֹϡ����ĵ�����
�ʴ�Ϊ�������У��������ױ����գ��������������ձ����ܵ�ĩ�˽�һ���۵�©�������հ����������������𰸣���
��8�����裺����þ������Ϊx�����ɳ�������Ϊy��
2NaOH+MgSO4�TNa2SO4+Mg��OH��2��
80 120 142 58
0.2m x y
=
=
��ã�x=0.3m y=0.145m
��������þ��Һ����������=
��100%=14.0%
����������þ��Һ����������Ϊ14.0%��
�ڷ�Ӧǰ����Һ����������Ŀ���䣬������������������þ�ĵμӲ�������������þ���������٣���������þ�ĵμӣ���Һ�е���������Ӳ������ӣ���Һ��ʼ�ղ���þ���ӣ�
�ʴ�Ϊ����ͼ��ʾ
�ʴ�Ϊ��cabd��
��2��ͨ������װ��������ʹ�������������װ��©����۲쵽װ���������Ա仯��������������ã����ƿ���Ҳർ��ˮ������������ʱˮ�������أ�
�ʴ�Ϊ���رշ�Һ©������������ƿ�����ƿ���Ҳർ��ˮ������������ʱˮ���������䣻
��3���ƾ������ͺ����Ȼ�̼��Ȼ�������백��������Ӧ��������ȴ�����ӷ����ӷ������������ʵ����Ӱ����һӷ�����������백����ˮ�Ӵ������ã��ټ�֮�ƾ�������ˮ���Ȼ�̼�ܶȱ�ˮ�����ܴﵽ�����Ŀ�ģ���ֲ���ͼȲ�����ˮҲ���ӷ������Ѱ�����ˮ���и��룻
�ʴ�Ϊ��C��
��4������ʵ���Ŀ�����ڲⶨ�������������������ռ����������壬��ˣ����ƿ�ڵ�ԭ�����岻�ڲ����ڣ�����Բ����������Ӱ�죻
�ʴ�Ϊ�����䣻
��5������̼����������������Һ������Ӧ��������������������������������Һ�����ܽ����ʧ����ˣ������й������ʱ��˵��ԭ�����к���̼��
�ʴ�Ϊ��̼��
��6���赪����������Ϊx
AlN+NaOH+H2O=NaAlO2+NH3��
41 17
y
| aL |
| 22.4L |
| 41 |
| y |
| 17 | ||
|
y=
| 41a |
| 22.4 |
��Ʒ��AlN����������=
| ||
| w |
| 4100a |
| 22.4w |
�ʴ�Ϊ��
| 4100a |
| 22.4w |
��7��������������ϡ��������ֵ�������ˣ���װ�ò���ȷ���������������������ڵ���ĩ������©��������Һ���ϣ��հ�����������ʱ���ձ���Һ���½�������Ӵ������Է�ֹϡ����ĵ�����
�ʴ�Ϊ�������У��������ױ����գ��������������ձ����ܵ�ĩ�˽�һ���۵�©�������հ����������������𰸣���
��8�����裺����þ������Ϊx�����ɳ�������Ϊy��
2NaOH+MgSO4�TNa2SO4+Mg��OH��2��
80 120 142 58
0.2m x y
| 80 |
| 0.2m |
| 120 |
| x |
| 80 |
| 0.2m |
| 58 |
| y |
��ã�x=0.3m y=0.145m
��������þ��Һ����������=
| 0.3m |
| 3m+0.145m-m |
����������þ��Һ����������Ϊ14.0%��
�ڷ�Ӧǰ����Һ����������Ŀ���䣬������������������þ�ĵμӲ�������������þ���������٣���������þ�ĵμӣ���Һ�е���������Ӳ������ӣ���Һ��ʼ�ղ���þ���ӣ�
�ʴ�Ϊ����ͼ��ʾ

���������û�ѧ�仯ǰ��Ԫ��������������䣬�����ð�����NԪ�ص�����������Ʒ�е�������������

��ϰ��ϵ�д�
�����Ŀ
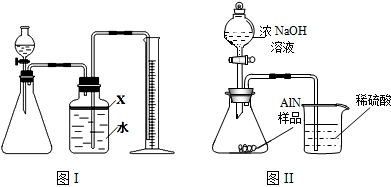
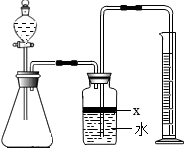 ��������AlN����һ�����������ϣ��㷺Ӧ���ڼ��ɵ�·��������ij�������к���̼�����������ʣ�ij��ѧ�о���ѧϰС����Ʋ����������ʵ�飮
��������AlN����һ�����������ϣ��㷺Ӧ���ڼ��ɵ�·��������ij�������к���̼�����������ʣ�ij��ѧ�о���ѧϰС����Ʋ����������ʵ�飮