��Ŀ����
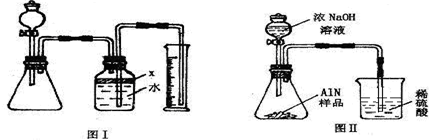
26����������AlN����һ�����������ϣ��㷺Ӧ���뼯�ɵ�·��������ij�������к���̼�����������ʣ�����ͼ���е�һЩװ�������м��飬ʹ��������Ʒ��NaOH��Һ��ӦAlN+NaOH+H2O=NaAlO2+NH3�������ݷ�Ӧ�������ɰ�����������ⶨ��Ʒ�еĵ�����������������������ʵ��������ȷ�����ʵijɷ֣�ʵ���е���������Բ��ƣ�
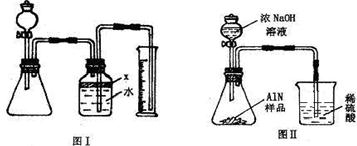
��1��ʵ���йز���Ϊ��a������ƿ�з���������AlN��Ʒ��b���ӷ�Һ©������ƿ�м��������ŨNaOH��c������װ�õ������ԣ�d���ⶨ�ռ���ˮ���������ȷ�IJ���˳��Ϊ
��2���������м��װ�������Եķ�����
��3�����ƿ�е��Լ�X��ѡ��
A ���� B �ƾ� C ֲ���� D CCl4
��4�����ƿ��Һ��û��װ�����Ϸ����������ռ䣩��ʵ����NH3�������
��5�����˸���ͼ��װ�ý���ͬ��ʵ�飬ͨ���ⶨ�ձ��������������ȷ����Ʒ��AlN����������������Ϊ�Ƿ���У�

��1��ʵ���йز���Ϊ��a������ƿ�з���������AlN��Ʒ��b���ӷ�Һ©������ƿ�м��������ŨNaOH��c������װ�õ������ԣ�d���ⶨ�ռ���ˮ���������ȷ�IJ���˳��Ϊ
c a b d
����2���������м��װ�������Եķ�����
�رշ�Һ©����������ˮ����������ƿ�����ƿ���Ҳർ��ˮ�����������ֺ�ˮ�����䣬֤�������Ժ�
����3�����ƿ�е��Լ�X��ѡ��
C
����ѡ��ı�ţ���A ���� B �ƾ� C ֲ���� D CCl4
��4�����ƿ��Һ��û��װ�����Ϸ����������ռ䣩��ʵ����NH3�������
����
����ƫ��ƫС�䣩����5�����˸���ͼ��װ�ý���ͬ��ʵ�飬ͨ���ⶨ�ձ��������������ȷ����Ʒ��AlN����������������Ϊ�Ƿ���У�
������
�����롰���С����������С�����ԭ�����������ױ����գ�������������
���Ľ��ķ���Ϊ�ձ����ܵ�ĩ�˽�һ���۵�©������©���ı�Ե�պ�û��Һ�����������հ���
����������1����ȡ����ʱ��Ϊ��ֹװ��©��Ӧ������װ�ú���������װ�������Լ�飬ȷ��װ�ò�©�������ȼӹ�����Һ���ԭ�����ҩƷ��������������ռ��������
��2���رշ�Һ©���Ļ�����װ�����γɷ�ջ����������װ�ý��м��ȣ�װ�����������������������������ã��ͻ�۲쵽���ƿ���Ҳർ��ˮ������������ʱˮ���������䣻
��3�������İ�����������ˮ��Ϊ��ֹ��������ˮ��Ҫ��������ˮ���룬���Ӧѡ�����백���������õ�Һ����Ϊ����Һ��ѡ�õ��Լ�Ӧ�Ǻ�ˮ�����ܣ����ܶȴ���ˮ�ģ�
��4������ʵ���Ŀ�����ڲⶨ�������������������ռ����������壬��ˣ����ƿ�ڵ�ԭ�����岻�ڲ�����Χ�ڣ�
��5�������������ᷴӦ��������臨�ʹϡ������Һ�������ӣ������ڷ�Ӧ��Ϊ���Ҷ���ʹϡ���ᵹ����������ձ���������ȷ��Ϊ�����������֣����ڵ���ĩ�˰�װ����©����ֹ������
��2���رշ�Һ©���Ļ�����װ�����γɷ�ջ����������װ�ý��м��ȣ�װ�����������������������������ã��ͻ�۲쵽���ƿ���Ҳർ��ˮ������������ʱˮ���������䣻
��3�������İ�����������ˮ��Ϊ��ֹ��������ˮ��Ҫ��������ˮ���룬���Ӧѡ�����백���������õ�Һ����Ϊ����Һ��ѡ�õ��Լ�Ӧ�Ǻ�ˮ�����ܣ����ܶȴ���ˮ�ģ�
��4������ʵ���Ŀ�����ڲⶨ�������������������ռ����������壬��ˣ����ƿ�ڵ�ԭ�����岻�ڲ�����Χ�ڣ�
��5�������������ᷴӦ��������臨�ʹϡ������Һ�������ӣ������ڷ�Ӧ��Ϊ���Ҷ���ʹϡ���ᵹ����������ձ���������ȷ��Ϊ�����������֣����ڵ���ĩ�˰�װ����©����ֹ������
����⣺��1��Ӧ�Ƚ���װ�������Լ��飬Ȼ�����μ������ҩƷ��Һ��ҩƷ�������������ų�ˮ�IJ�����ȷ���������������
�ʴ�Ϊ��c a b d��
��2��ͨ���Ȼ���������ʹװ��������ʹ�������������װ��©����۲쵽װ���������Ա仯��������������ã����ƿ���Ҳർ��ˮ�����������ֺ�ˮ�����䣻
�ʴ�Ϊ���رշ�Һ©����������ˮ����������ƿ�����ƿ���Ҳർ��ˮ�����������ֺ�ˮ�����䣬֤�������Ժã�
��3���ƾ���������Ȼ�������백��������Ӧ��������ȴ�����ӷ����ӷ������������ʵ����Ӱ����һӷ�����������백����ˮ�Ӵ������ã�ͬʱ���ھƾ�������ˮ��Ҳ���ܴﵽ�����Ŀ�ģ�CCl4�ܶȴ���ˮ�������������ã���ֲ���ͼȲ�����ˮ���ܶ�С��ˮҲ���ӷ������Ѱ�����ˮ���и��룻
�ʴ�Ϊ��C��
��4������ʵ���Ŀ�����ڲⶨ�������������������ռ����������壬��ˣ����ƿ�ڵ�ԭ�����岻�ڲ����ڣ�����Բ����������Ӱ�죻
�ʴ�Ϊ�����䣻
��5��������������ϡ��������ֵ�������ˣ���װ�ò���ȷ���������������������ڵ���ĩ������©��������Һ���ϣ��հ�����������ʱ���ձ���Һ���½�������Ӵ������Է�ֹϡ����ĵ�����
�ʴ�Ϊ�������У��������ױ����գ��������������ձ����ܵ�ĩ�˽�һ���۵�©������©���ı�Ե�պ�û��Һ�����������հ�����
�ʴ�Ϊ��c a b d��
��2��ͨ���Ȼ���������ʹװ��������ʹ�������������װ��©����۲쵽װ���������Ա仯��������������ã����ƿ���Ҳർ��ˮ�����������ֺ�ˮ�����䣻
�ʴ�Ϊ���رշ�Һ©����������ˮ����������ƿ�����ƿ���Ҳർ��ˮ�����������ֺ�ˮ�����䣬֤�������Ժã�
��3���ƾ���������Ȼ�������백��������Ӧ��������ȴ�����ӷ����ӷ������������ʵ����Ӱ����һӷ�����������백����ˮ�Ӵ������ã�ͬʱ���ھƾ�������ˮ��Ҳ���ܴﵽ�����Ŀ�ģ�CCl4�ܶȴ���ˮ�������������ã���ֲ���ͼȲ�����ˮ���ܶ�С��ˮҲ���ӷ������Ѱ�����ˮ���и��룻
�ʴ�Ϊ��C��
��4������ʵ���Ŀ�����ڲⶨ�������������������ռ����������壬��ˣ����ƿ�ڵ�ԭ�����岻�ڲ����ڣ�����Բ����������Ӱ�죻
�ʴ�Ϊ�����䣻
��5��������������ϡ��������ֵ�������ˣ���װ�ò���ȷ���������������������ڵ���ĩ������©��������Һ���ϣ��հ�����������ʱ���ձ���Һ���½�������Ӵ������Է�ֹϡ����ĵ�����
�ʴ�Ϊ�������У��������ױ����գ��������������ձ����ܵ�ĩ�˽�һ���۵�©������©���ı�Ե�պ�û��Һ�����������հ�����
����������װ�õ������ԵIJ����ǣ���һ����װ���ܱգ�����ť�Ĺرգ��ʹ�����ͬ����ˮ�ܷ⣩���ڶ���������ѹǿ����²�������ֽ������ȡ��þƾ����ȡ���μ�Һ�����Һ���ȷ���������������������

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
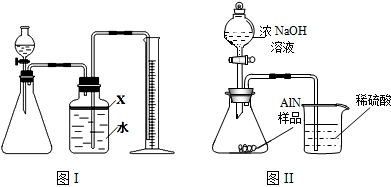
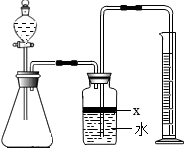 ��������AlN����һ�����������ϣ��㷺Ӧ���ڼ��ɵ�·��������ij�������к���̼�����������ʣ�ij��ѧ�о���ѧϰС����Ʋ����������ʵ�飮
��������AlN����һ�����������ϣ��㷺Ӧ���ڼ��ɵ�·��������ij�������к���̼�����������ʣ�ij��ѧ�о���ѧϰС����Ʋ����������ʵ�飮