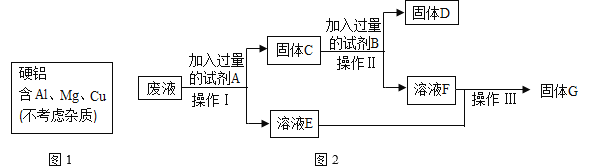
题目内容
【题目】化学的发展与人类的生活、生产息息相关。
(1)芋头皮内含有碱性黏液,皮肤沾上会出现瘙痒,可用厨房中_____来止痒。
(2)医疗上常用3%的过氧化氢溶液进行伤口消毒,消毒时常看到有气泡产生,产生这一现象的原理是_____(用化学方程式表示)。
(3)金属镁在空气中燃烧除了生成氧化镁,还可以生成氮化镁(Mg3N2)固体,氮化镁与水反应,生成氢氧化镁和氨气,氨气可以用于制得固体化肥氯化铵,请写出氮化镁与水反应的化学方程式_____。
(4)酒精是清洁燃料之一,写出酒精完全燃烧的化学方程式_____。
【答案】食醋 2H2O2=2H2O+O2↑ Mg3N2+6H2O=3Mg(OH)2+2NH3↑ 
【解析】
(1)芋头皮内含有碱性黏液,皮肤沾上会出现瘙痒,所选的物质应该能与碱性反应且对皮肤无副作用,厨房中的食醋符合要求。
(2)过氧化氢溶液常温下会缓慢分解为水和氧气,化学方程式为:2H2O2=2H2O+O2↑。
(3)氮化镁固体与水反应生成氢氧化镁和氨气,写出反应物与生成物配平即可,化学方程式为:Mg3N2+6H2O=3Mg(OH)2+2NH3↑。
(4)酒精燃烧需要消耗氧气,生成物为二氧化碳和水,反应的化学方程式为: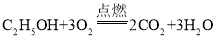 。
。

 快乐暑假暑假能力自测中西书局系列答案
快乐暑假暑假能力自测中西书局系列答案【题目】黄铜是一种重要的金属,它是铜和锌的合金,可用来制造机器、电器零件及日常用品。为了测定黄铜样品的组成,取五份样品分别加稀硫酸反应,其实验数据记录如下:
样品 | 第1份 | 第2份 | 第3份 | 第4份 | 第5份 |
取样品质量(g) | 40.0 | 40.0 | 40.0 | 40.0 | 40.0 |
取稀硫酸质量(g) | 30.0 | 60.0 | 90.0 | 120.0 | 150.0 |
产生气体质量(g) | 0.3 | 0.6 | 0.9 | 1.0 | 1.0 |
按要求回答下列问题:
(1)根据实验数据分析,从第________份开始,金属已经反应完全了。
(2)列式计算该黄铜样品中金属锌的质量分数。(要求写出计算过程)