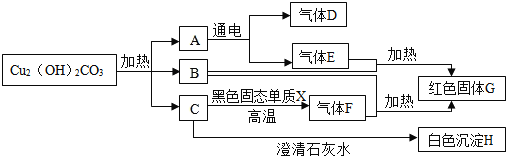
��Ŀ����
����Ŀ��С��ȥ��ժ��ݮ��
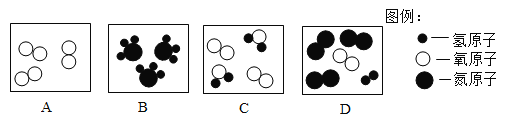
��1����ݮ�������õ����ϱ�Ĥ����_____������ţ���ͬ����
A �л��ϳɲ��� B ��������
��2���÷��ͺ��ţ�������ϣ��ֳ��IJ�ÿ�ִ����𡣷��ͺ��ţ�̺���C��H��O��N��Ԫ�ء�����ţ�̡��൱��ʩ����_____��
A ���� B �� C �ط�
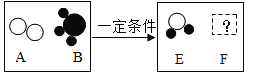
��3��ʳ�ò�ݮǰ����Ҫ�á�ʳ����ϴ������5��10���ӣ�������ˮϴ����С����1g�������ƺ�2Lˮ���Ƴɡ�ʳ����ϴ�����������ʵ����������ļ���ʽΪ_____������ʯ���Ƶ����������������ķ�Ӧ_____��

���𰸡�A A ![]() CaO+H2O=Ca(OH)2
CaO+H2O=Ca(OH)2
��������
��1�����������л��ϳɲ��ϣ���ѡA��
��2��ţ���к��е�Ԫ�أ����ڵ��ʣ���ѡA��
��3��1g������������2Lˮ��ȫ���ܽ⣬���������Ƶ�������������Ϊ![]() ����������ˮ��Ӧ������������,�ʷ�Ӧ�Ļ�ѧ����ʽΪCaO+H2O=Ca(OH)2��
����������ˮ��Ӧ������������,�ʷ�Ӧ�Ļ�ѧ����ʽΪCaO+H2O=Ca(OH)2��

 ѧ���쳵��������������������ϵ�д�
ѧ���쳵��������������������ϵ�д�����Ŀ������ʵ�������ͼ��ʾ�仯���Ƶ��ǣ�������
��� | ʵ�� | ������ | ������ |
A | ��һ��������������Һ�еμ�ϡ���� | ϡ��������� | ˮ������ |
B | ����һ����������ع��� | ʱ�� | ʣ���������Ԫ�ص����� |
C | ��һ���������������Һ�м�ˮ | ��ˮ������ | ���ʵ��������� |
D | ��һ����þ���м���ϡ���� | ϡ��������� | þ�۵����� |

A.AB.BC.CD.D