��Ŀ����
����Ŀ��ij��ѧѧϰС������ʦ��ָ���£���ɫ�۱ʵijɷֽ���̽����
��������⣩��ɫ�۱ʵ���Ҫ�ɷ���ʲô�أ���ʦ��ʾ��һ�ֳ����ĸ��������������ȶ���ճ�ϼ��Ƴɵġ�
����������裩ͬѧ�ֱ��������²��룺
A ̼��� B �������� C ����� D �Ȼ��� E �����
ͬѧ�Ǹ�����ʦ��ʾ�����ۣ���������B�IJ��룬����Ϊ������________��
���������ϣ�
��1�����������Ȼ����ʯ������Ҫ�ɷ֣�Ϊ��ɫ���壬����ˮ��
��2�������¼������ʵ��ܽ��
���� | ̼��� | �Ȼ��� | ����� | ����� |
�ܽ��/g | 0��0013 | 74��5 | 0��3 | 138 |
������ʵ�飩
ʵ����� | ���� | ���� | |
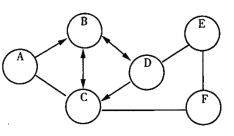
�� | ȡ������ɫ�۱��������Թ��У�����________ | ������ | A������ |
�� | ȡ������ɫ�۱��������ձ��У���������ˮ������ | ����û�����Լ��� | ________������ |
�� | ���ڽ���________�������õ���ҺA���壬����Һ�м��� ____________��Һ���ټ�������ϡ���� | ������ɫ��������������ʧ | C���� |
��д�����з�����Ӧ�Ļ�ѧ����ʽ___________��
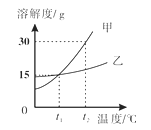
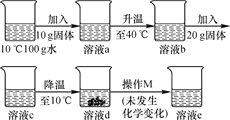
����չ���죩Ϊ�˲ⶨ��ʯ�����ɣ�CaSO4��xH2O�������ⶨx��ֵ����������ʵ�飺�����ᾧˮ������Ʒ��������м��ȣ�����ǰ�ͼ��Ⱥ��г���������ʵ����������ӣ����ȵ�ʱ�䲻���ӳ���������ʵ���н������������£����������ݻ��Ƶ�ͼ����ͼ��ʾ��
�������ϣ����ᾧˮ������������������ʽ���ڼ��ȹ����У��ֲ�ʧȥ�ᾧˮ�����յõ�����ƹ��塣
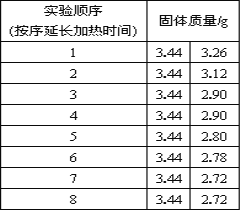

��1��AB�ι������������ԭ��__________��
��2������ʵ�����ݣ�����x= ________��
��3��ͼ��CD�ι������ʵĻ�ѧʽ _____________��
���𰸡����������Ǽ�������� ϡ���� DE ���� �Ȼ�����BaCl2�� CaSO4+ BaCl2=BaSO4��+CaCl2 δ�ﵽ�ֽ��¶� 2 2CaSO4��H2O
��������
���������Ǽ���Σ�����ƺ��Ȼ�����Ӧ�������ᱵ�������Ȼ��ƣ�ϡ�����̼��Ʒ�Ӧ�����Ȼ��ƺ�ˮ��
[���������]
��ɫ�۱ʵ���Ҫ�ɷ���һ�ֳ����ĸ��������������ȶ���ճ�ϼ��Ƴɵ�,���������Ǽ��������,��B�IJ��벻��ȷ��
[����ʵ��]
ʵ����� | ���� | ���� | |
�� | ȡ������ɫ�۱��������Թ��У�����ϡ���� | ������ | A������ |
�� | ȡ������ɫ�۱��������ձ��У���������ˮ������ | ����û�����Լ��� | DE������ |
�� | ���ڽ��й��˲������õ���ҹA���壬����Һ�м����Ȼ�����Һ���ټ�������ϡ���� | ������ɫ��������������ʧ | C���� |
���з����ķ�Ӧ������ƺ��Ȼ�����Ӧ�������ᱵ�������Ȼ��ƣ���ѧ����ʽΪ![]()
[��չ����]
��1��AB�ι������������ԭ��δ�ﵽ�ֽ��¶ȡ�
��2��ʯ����ȷֽ���ٵ���������ˮ������
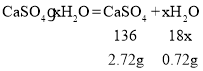
![]()
![]()
��3��ͼ��CD��ʱ��ʯ�������Ϊ2.90g������ͼ�����ݿ�֪����ͷ����û�䣬˵����û���˽ᾧˮ�������������Ϊ2.72g����ˮ������Ϊ![]() �����������ˮ��������Ϊ
�����������ˮ��������Ϊ![]() �����������ˮ����Է�������֮��Ϊ
�����������ˮ����Է�������֮��Ϊ![]() ���������ǰ��Ӧ��д��2���ʹ������ʵĻ�ѧʽ2CaSO4��H2O��
���������ǰ��Ӧ��д��2���ʹ������ʵĻ�ѧʽ2CaSO4��H2O��

����Ŀ����һ�������ĩ�����к���̼���ơ��������ơ�̼��ơ���ʯ�ҡ��Ȼ����е��������ʡ�ij��ȤС��Ϊȷ���������Ʋ���������ʵ�顣
��ʵ��һ������ͬѧ��������̽��������ɱ��пհס�
ʵ����� | ʵ������ | ʵ����ۼ����� |
��ȡ�����ù����ĩ���ձ��У�����������ˮ�ܽ⡢���� | ��ĩ�����ܽ⣬�õ���ɫ��������ɫ��Һ | ������һ������_____ |
������Һ�еμ���ɫ��̪��Һ | ��Һ��� | ��Һ�п��ܺ���_____���ʣ������������������������������� |
�������������Һ�μ�����ϡ���� | _____ | ԭ�����ĩ��һ������Na2CO3 |
��ʵ���������ͬѧ��������̽����
ȡ21.0g�ù����ĩ������160.0g 10%��ϡ���ᣬǡ����ȫ��Ӧ��ʵ���ò���8.8g������̼������Ӧ����Һ���������ᾧ���õ����壬�ⶨ���ù����к�16.0g��Ԫ�ء�
���ۺϼס�������ͬѧ��ʵ����з�������գ�
��1������ʵ����һ�������Ļ�ѧ��Ӧ�ǣ�Na2CO3+2HCl�T2NaCl+H2O+CO2��
��2�������ĩ������г�Na2CO3�⣬һ�������е�������_____�����ܺ��е�������_____������д��ѧʽ��