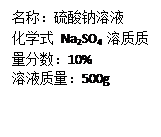��Ŀ����
�����벻����ѧ��
��ʳ����Լ����3%-5%�Ĵ��ᣨC2H4O2����������_________��Ԫ����ɡ�C2H4O2��Ħ������Ϊ_________��0.5mol C2H4O2������Լ����_________����ԭ�ӡ�
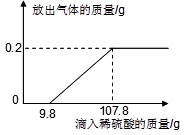
����ͼ�ǵ��ˮ��װ�ã�д��ˮ���Ļ�ѧ����ʽ__________________����������ķ��Ӹ�������_______��������������ʵ�������֮����ȡ����³�ѹ�£����������������ܽ�ȷֱ�Ϊ1.63��10-3g/100gˮ��4.34��10-3g/100gˮ���ڵ������У��ס����������������ȿ���________������ڡ���С�ڡ�����
��ʳ����Լ����3%-5%�Ĵ��ᣨC2H4O2����������_________��Ԫ����ɡ�C2H4O2��Ħ������Ϊ_________��0.5mol C2H4O2������Լ����_________����ԭ�ӡ�
����ͼ�ǵ��ˮ��װ�ã�д��ˮ���Ļ�ѧ����ʽ__________________����������ķ��Ӹ�������_______��������������ʵ�������֮����ȡ����³�ѹ�£����������������ܽ�ȷֱ�Ϊ1.63��10-3g/100gˮ��4.34��10-3g/100gˮ���ڵ������У��ס����������������ȿ���________������ڡ���С�ڡ�����

3��60g/mol��6.02��1023��2H2O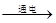 2H2��+O2�������ʵ���������
2H2��+O2�������ʵ���������
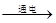 2H2��+O2�������ʵ���������
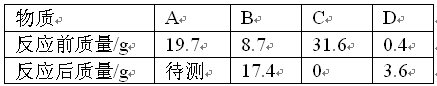
2H2��+O2�������ʵ������������ٸ��ݴ���Ļ�ѧʽC2H4O2����������C��H��O������Ԫ����ɣ������Ħ������=12��2+1��4+16��2=60g/mol��0.5mol C2H4O2������Լ������ԭ�Ӹ���=6.02��1023��0.5��2���T6.02��1023����
�ʴ�Ϊ��3��60g/mol��6.02��1023��
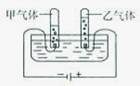
�ڷ�Ӧ��Ϊˮ��������Ϊ�������������ٸ�����С����������ƽ���ɣ���ˮ���Ļ�ѧ����ʽΪ2H2O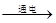 2H2��+O2�����ڱ�״���·��Ӹ����������ʵ��������ȣ�������������ˮ�е��ܽ����ϴ������������������������ȿ��ܴ���2��1
2H2��+O2�����ڱ�״���·��Ӹ����������ʵ��������ȣ�������������ˮ�е��ܽ����ϴ������������������������ȿ��ܴ���2��1
�ʴ�Ϊ��2H2O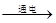 2H2��+O2�������ʵ��������ڣ�
2H2��+O2�������ʵ��������ڣ�
�ʴ�Ϊ��3��60g/mol��6.02��1023��
�ڷ�Ӧ��Ϊˮ��������Ϊ�������������ٸ�����С����������ƽ���ɣ���ˮ���Ļ�ѧ����ʽΪ2H2O
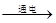 2H2��+O2�����ڱ�״���·��Ӹ����������ʵ��������ȣ�������������ˮ�е��ܽ����ϴ������������������������ȿ��ܴ���2��1
2H2��+O2�����ڱ�״���·��Ӹ����������ʵ��������ȣ�������������ˮ�е��ܽ����ϴ������������������������ȿ��ܴ���2��1�ʴ�Ϊ��2H2O
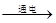 2H2��+O2�������ʵ��������ڣ�
2H2��+O2�������ʵ��������ڣ�
��ϰ��ϵ�д�
�����Ŀ