��Ŀ����
����Ŀ������ͭ�ڷ�֯��ӡˢ����ҵ�й㷺��;��
һ��̽������ͭ���Ʊ�
��ҵ�Ͽ���ͭм��������ϡ������80���������Ʊ�����ͭ������ͼ��ij�о���ѧϰС��ʵ��
��ģ�ҵ�Ʊ�����ͭ��װ�á�
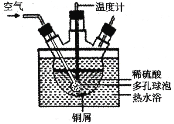
��1��������ݵ�����Ϊ_________�÷�Ӧ�Ļ�ѧ����ʽΪ_________
����̽������ͭ������
ʵ��һ���о���ѧϰС��ͬѧ������Ͷ������ͭ��Һ�У��������ɺ�ɫ�������ʵ�ͬʱ�н϶�����ݷų�����һ������ͬѧ�ǵ�̽�����������ɵ���ʲô���壿
������룩���������Ԫ�صĽǶȣ��ų������������SO2��O2��H2�е�һ�ֻ��֡�
���������ϣ������Ը��������Һ���Ϻ�ɫ��SO2��ʹ���Ը��������Һ��ɫ��
��O2��4KI��4HC��2I2��4KCl��2H2O��I2Ϊ�ⵥ�ʣ���������Һ������
��������ƣ������������룬�о���С��ͬѧ�ֱ���������·�����
��2����ͬѧΪȷ���Ƿ���SO2����������ͨ�����Ը��������Һ�У���Һ��ɫδ�����仯�����������______SO2����������������������
��3����ͬѧ��Ϊֻ��O2���������________________�����顣ʵ��֤����ͬѧ�Ĺ۵㲻��ȷ
��4����ͬѧΪ�ж��Ƿ���O2��ͬʱȷ������ɷ֣���������µ�ʵ��װ�ã�
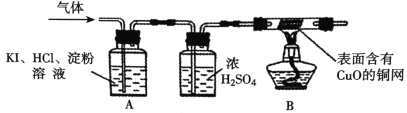
��ʵ����ۣ�A�й۲쵽________________��֤����O2��B�й۲쵽____________��֤����������H2��
��ʵ�鷴˼�������Ϊ��ͬѧ�ڼ���֮ǰ��Ӧ��������_____________��ȷ����ȫ��
ʵ�����С��ͬѧ�ֽ�������Na2CO3��Һ���뵽CuSO4��Һ�еõ�һ������ɫ���壬������ɫ����������������CuO��H2O��CO2���Ӷ�ȷ����ɷ�Ϊ��ʽ̼��ͭ����ѧʽ�ɱ�ʾΪCuX(OH)YCOZ ��
������̽����Ϊ��һ��ȷ����ʽ̼��ͭ�Ļ�ѧʽ��С��ͬѧȷ��ȡ����ɫ����32��0g��������װ�ã��г�����δ����������ʵ�顣
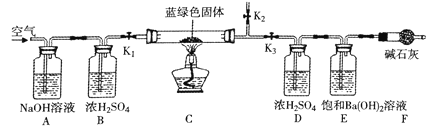
����1����ֹˮ��K1��K2���ر�K3��ͨ�������һ��ʱ���ر�ֹˮ��K1��K2����K3��
����2����ȼ�ƾ��ƣ���C��Ӳ�ʲ������й�����ȫ��ɺ�ɫ��ĩ����ֹˮ��K1������ͨ���������ȴ�����¡�
�����������ۣ�
��5��Aװ�õ�������_________
��6��Ӳ�ʲ������й�����ȫ��ɺ�ɫ��ĩ�����ͨ�������Ŀ����_________
�����ݴ�����
��7����ʵ���|������Ӧǰ��Dװ������3��6g��E�в�����ɫ����������Ϊ19��7g���������ɫ����Ļ�ѧʽΪ_________________���г�������̣���
��8��E����Ba(OH)2������Һ�������ʯ��ˮ��ԭ����:
��BaCO3����Է�����������CaCO3�����ɳ����������������С
��_________
���𰸡�������������Һ�ĽӴ������ʹ��Ӧ��ֽ��� 2Cu+O2+2H2SO4=2CuSO4+2H2O �� �����ǵ�ľ�� ��Һδ������ɫ�������� ͭ�������� �鴿 ���տ����е�CO2 ��װ���зֽ������CO2��ˮ����ȫ������D��Eװ���У���ȫ���գ�����ʵ����� Cu3(OH)4CO3 Ba(OH)2���ܽ�ȴ���Ca(OH)2�����Գ������CO2
��������
��1����Ϊ������μӷ�Ӧ���ʲ��ö��������������������Һ�ĽӴ������ʹ��Ӧ��ֽ��У���Ӧ����ͭ���������������������ͭ����һ�ֲ������Ԫ���غ��֪��ˮ����Ӧ�Ļ�ѧ����ʽΪ��2Cu+O2+2H2SO4=2CuSO4+2H2O�����������������Һ�ĽӴ������ʹ��Ӧ��ֽ��У�2Cu+O2+2H2SO4=2CuSO4+2H2O
��2������������ʹ���Ը��������ɫ������Һ��ɫ����֤����SO2������Һ�����Ա仯����֤��û��SO2����ͬѧΪȷ���Ƿ���SO2����������ͨ�����Ը��������Һ�У���Һ��ɫδ�����仯�������������SO2�������
��3�����Բ��ô����ǵ�ľ����������������ͬѧ��Ϊֻ��O2�����ô����ǵ�ľ�������顣��ľ����ȼ��֤��������������ȼ֤��û����������������ǵ�ľ��
��4��[ʵ�����] ������ͨ�뺬������͵⻯�صĵ�����Һ�У�������Ӧ��O2��4KI��4HCl��2I2��4KCl��2H2O��I2Ϊ�ⵥ�ʣ���������Һ������B������ͭ�ڼ�������¿��Ա�������ԭ������ͭ���ʺ�ˮ��A�й۲쵽��Һδ������ɫ��������֤����O2��B�й۲쵽ͭ�������죬֤����������H2�������Һδ������ɫ��������ͭ��������
[ʵ�鷴˼]�ڼ��ȿ�ȼ������֮ǰ��Ϊ��ʵ�鰲ȫ����ֹ������ը�������Ϊ��ͬѧ�ڼ���֮ǰ��Ӧ�������ȼ�������Ĵ��ȣ���ȷ����ȫ������鴿
[����������]
��5�����������������ƣ���ͨ��Ũ���ᣬĿ����Ϊ�˳�ȥ�����еĶ�����̼��ˮ��������Aװ�õ����������տ����е�CO2��������տ����е�CO2
��6����Cװ��Ӳ�ʲ������м�������ɫ���壬�ֽ���������ͭ��������̼��ˮ����Ũ�����������ɵ�ˮ�֣�������������Һ�������ɵĶ�����̼��������ȫ��ɺ�ɫ��ĩ�����ͨ�������Ŀ���ǽ�װ���зֽ������CO2��ˮ����ȫ������D��Eװ���У���ȫ���գ�����ʵ���������װ���зֽ������CO2��ˮ����ȫ������D��Eװ���У���ȫ���գ�����ʵ�����
��7����32.0g����ֽ����CO2������Ϊx��
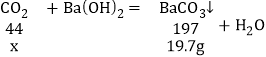
![]() ����ã�
����ã�![]()
�ֽ����CO2������Ϊ4.4g��m��C����1.2g
Dװ������3.6g��m��H2O����3.6g��m��H����0.4g
32.0g����ֽ����ɵ�m��CuO����32.0g��3.6g��4.4g��24.0g��m��Cu����19.2g
32.0g������m��O����32.0g��19.2g��1.2g��0.4g��11.2g
32.0g����Cu��C��O��H����Ԫ�ص�������Ϊ19.2��1.2��1.2��0.4
32.0g����Cu��C��O��H����Ԫ�ص�ԭ�Ӹ�����Ϊ3��1��7��4
��ѧʽΪCu3(OH)4CO3�����Cu3(OH)4CO3
��8��E����Ba(OH)2������Һ�������ʯ��ˮ��ԭ���ǣ�BaCO3����Է��������Ƚϴ���ʱ���������С��Ca(OH)2���ܽ���С����Һ��Ũ�Ȳ����ԱȽ������Զ�����̼�����ղ���֣���Ba(OH)2���ܽ�Ƚϴ�Ũ�Ƚϴ��Գ������CO2�����Ba(OH)2���ܽ�ȴ���Ca(OH)2�����Գ������CO2

����Ŀ��ͨ���Ƚ���ͬʱ���ڲ��������������̽��Ӱ���������ֽ����ʵ����أ�
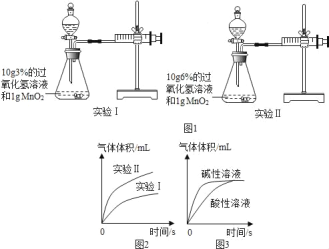
��1����ͼ1װ�ý���ʵ�鲢��������ͼ��ͼ2��
д��ʵ��I�Ļ�ѧ��Ӧ����ʽ��_____������ͼ1��ͼ2��Ϣ��֪�������ضԹ�������ֽ����ʵ�Ӱ���ǣ�_____��
��2��̽���¶ȼ�����������Ӱ�죮�벹��ʵ����е����ݣ�
ʵ���� | �¶� | ���� | ����������Һ��������Ũ�� |
ʵ��� | 50�� | 1g�������� | 10g��3% |
ʵ��� | 25�� | 2g�������� | 10g��3% |
ʵ��� | _____ | _____ | 10g��3% |
��3����Һ����ԶԹ�������ķֽ�����Ҳ��Ӱ�죬��ͼ3��
������ͼ3���ɵó��Ľ����ǣ�_____��
�����ϱ���������������BaO2�������ᣨH2SO4����Һ��Ӧ��������ȡH2O2��ͬʱ����һ�ְ�ɫ�������ᱵ��BaSO4���÷�Ӧ�Ļ�ѧ����ʽΪ��_____���ӷ�Ӧ��Ļ�����г�ȥ���ᱵ������ʵ������ǣ�_____���������н��ۿ�֪������������Ӧ��ȡH2O2ʱ�������������Ӧ��_____�������������������
����Ŀ����������ͼ���е���Ϣ��ѧϰ��ѧ��һ����Ҫ������
��1��������Ȼ��ƺ�̼�����ڲ�ͬ�¶�ʱ���ܽ�ȣ����ݴ˱��ش�
�¶�/�� | 10 | 20 | 30 | 40 | |
�ܽ��/g | �Ȼ��� | 35.8 | 36.0 | 36.3 | 36.6 |
̼���� | 12.2 | 21.8 | 39.7 | 53.2 | |
��40��ʱ���Ȼ��Ƶ��ܽ��Ϊ_____g��
��̼���Ƶ��ܽ�����¶ȵ����߶�_____������������������С��������20��ʱ����100g��ˮ����30g̼�����У���ֽ����õ�����_____������������Һ��������������Һ��������������Һ���µ�30�棬����Һ��������������Ϊ_____����������ȷ��0.1%����
��10��ʱ���ֱ����Ʊ����������ʵı�����Һ��������������������С��������_____��
��2����������ϡ�������һ����������ͭ������У�д�����з�Ӧ�Ļ�ѧ����ʽ_____����ͼ��ʵ��������������ʣ�����������淴Ӧʱ��ı仯��ϵ�����б�ʾ��ȷ����_____�����ţ���