��Ŀ����
ij�о���ѧϰС���ȡ��NaCl��Na2CO3����25g���������Ƴ���Һ������������μ���������7.3%��ϡ���ᣬʹ������ȫ�ų������ռ��� 8.8gCO2���壮��l������ԭ������Na2CO3�����������������������������
��2���±�Ϊ�о���ѧϰС�����������ƵĻ��Һ�з�������μ��루�ӱ������������¼�IJ������ݣ�����������֪Na2CO3��HCl��Ӧ���Է��������У�Na2CO3+HCl��NaCl+NaHCO3��I����NaHCO3+HCl=NaCl+H2O+CO2����
������ɱ�����δ��IJ��֣�
| ʵ����� | ÿ�β�����CO2��������g�� |
| ��һ������μ�����100g | |
| �ڶ�������μ�����100g | 8.8 |
| ��������������100g | 0 |
��������1��������ȫ��Ӧʱ���������Ϊ8.8g����������̼����������̼���������ᷴӦ�Ļ�ѧ��Ӧ����ʽ���м���õ�̼���Ƽ�����������
��2������Na2CO3��HCl��Ӧ���Է��������У�Na2CO3+HCl=NaCl+NaHCO3��NaHCO3+HCl=NaCl+H2O+CO2�����Ӳμӷ�Ӧ�����ʵ����������������Ļ�ѧ��Ӧ�����ж��Ƿ�������壮
��2������Na2CO3��HCl��Ӧ���Է��������У�Na2CO3+HCl=NaCl+NaHCO3��NaHCO3+HCl=NaCl+H2O+CO2�����Ӳμӷ�Ӧ�����ʵ����������������Ļ�ѧ��Ӧ�����ж��Ƿ�������壮
����⣺��l����ԭ������Na2CO3������Ϊx�����������������Ϊy����
Na2CO3+2HCl=2NaCl+H2O+CO2����
106 73 44
x y��7.3% 8.8g
=
��
=
���x=21.2g��y=200g��
̼���Ƶ���������Ϊ
��100%=84.8%��
��̼���Ƶ���������Ϊ84.8%�����������������Ϊ200g��
��2������Na2CO3+HCl=NaCl+NaHCO3��NaHCO3+HCl=NaCl+H2O+CO2����֪������100g����ʱֻ������һ����Ӧ��
�ڷ�ӦNa2CO3+HCl=NaCl+NaHCO3��
106 36.5
21.2 100g��7.3%
=
����ʱ������������̼���壬�����������Ϊ0���ټ�100g����ǡ����ȫ��Ӧ���õ�8.8g������̼���壬�ټ������ᷴӦ���������������μ�����õ���������Ϊ0���ʴ�Ϊ��
�ڹ�ϵͼ��
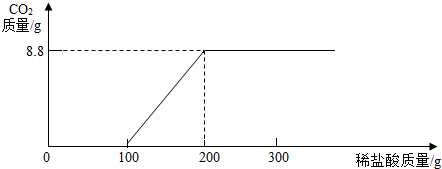
Na2CO3+2HCl=2NaCl+H2O+CO2����
106 73 44
x y��7.3% 8.8g
| 106 |
| x |
| 44 |
| 8.8g |
| 73 |
| y��7.3% |
| 44 |
| 8.8g |
���x=21.2g��y=200g��
̼���Ƶ���������Ϊ
| 21.2g |
| 25g |
��̼���Ƶ���������Ϊ84.8%�����������������Ϊ200g��
��2������Na2CO3+HCl=NaCl+NaHCO3��NaHCO3+HCl=NaCl+H2O+CO2����֪������100g����ʱֻ������һ����Ӧ��
�ڷ�ӦNa2CO3+HCl=NaCl+NaHCO3��
106 36.5
21.2 100g��7.3%
| 106 |
| 21.2 |
| 36.5 |
| 100g��7.3% |
| ʵ����� | ÿ�β�����CO2��������g�� |
| ��һ������μ�����100g | 0 |
| �ڶ�������μ�����100g | |
| ��������������100g |

������������ѣ����黯ѧ��Ӧ����ʽ�ļ��㣬ѧ��Ӧע�ⷴӦ�ķֲ����У���ע�ⷴӦ��ͼ��Ķ�Ӧ��ϵ��

��ϰ��ϵ�д�
�����Ŀ
Ϊ�˲ⶨ��ͭ��ͭ��п�Ͻ𣩵���ɣ�ij�о���ѧϰС���ȡ����Ʒ10g�����ձ��У���80g9.8%��ϡ������Ĵμ�����ձ��У���ַ�Ӧ���ʣ������������ݼ�¼���£�
�Լ��㣺
��1����Ʒ��ͭ������������ ��
��2����Ӧ�����ĵ�������Һ������
��3�������μ��������ַ�Ӧ��������Һ�����ʵ����������� ��
| ���� | 1 | 2 | 3 | 4 |
| ����ϡ���������/g | 20.0 | 20.0 | 20.0 | 20.0 |
| ʣ����������/g | 8.7 | 7.4 | 6.1 | 5.8 |
��1����Ʒ��ͭ������������
��2����Ӧ�����ĵ�������Һ������
��3�������μ��������ַ�Ӧ��������Һ�����ʵ�����������
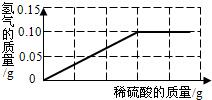 Ϊ�˲ⶨ��ͭ��ͭ��п�Ͻ𣩵���ɣ�ij�о���ѧϰС���ȡ����Ʒ10g����������μ���9.8%��ϡ���ᣬ��Ӧ���������ɵ�����������������Һ��������ϵ��ͼ��ʾ���Լ��㣺
Ϊ�˲ⶨ��ͭ��ͭ��п�Ͻ𣩵���ɣ�ij�о���ѧϰС���ȡ����Ʒ10g����������μ���9.8%��ϡ���ᣬ��Ӧ���������ɵ�����������������Һ��������ϵ��ͼ��ʾ���Լ��㣺 A��B���ֹ������ʵ��ܽ��������ͼ��ʾ��������������⣺
A��B���ֹ������ʵ��ܽ��������ͼ��ʾ��������������⣺