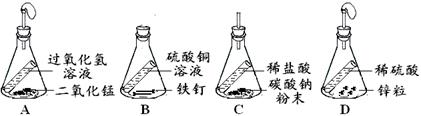
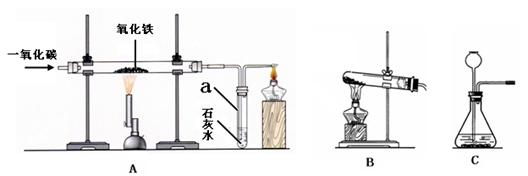
��Ŀ����
�й�������ʾ���ҹ���������Ҫ����Ϊ����ȼ�պ������ߵ�ú���γɵģ����ҹ�����Դ�ṹ�У�ȼúԼռ70%�������⣬���ֻ������ŷŵ�β��Ҳ���γ��������Ҫԭ��
��������⡿ͨ��ʵ��֤��ú�к���̼Ԫ�غ���Ԫ�ء�
���������ϡ��١�����������ʹ���������Һ��ɫ�����Ϻ�ɫ��Ϊ��ɫ�����÷�Ӧ�Ļ�ѧ����ʽΪ��5SO2 + 2KMnO4 + 2H2O="==" K2SO4 + 2MnSO4 + 2 �������������ѧ����ʽ�����һ�����ʵĻ�ѧʽӡˢ����������Ʋ��仯ѧʽ�� ��
�ڡ���������Ͷ�����̼һ����Ҳ��ʹ����ʯ��ˮ����ǡ�������д�÷�Ӧ�Ļ�ѧ����ʽ�� ��
�����������ϣ���ͬѧ����ͬѧ�ֱ����ʵ�鷽������̽����
��1����ͬѧ��
������ʵ�顿��ͬѧ����������ͼ��ʾA��B����ʵ�飺

���۲�����A��ϡ���������Һ��ɫ��B�г���ʯ��ˮ����ǡ�
���ó����ۡ�úȼ�����ɶ�������Ͷ�����̼��֤��ú�к���̼Ԫ�غ���Ԫ�ء�
����Ϊ��ʵ�鷽���Ƿ�������������ɣ� ��
��2����ͬѧ��
������ʵ�顿��ͬѧ����������ͼ��ʾʵ�飨����װ����ͼ����ȥ����
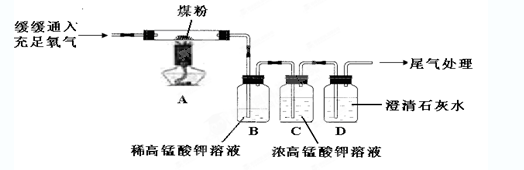
Bװ�õ������� ��
���۲�����
�����B��C��D�����
���ó����ۡ�úȼ�����ɶ�������Ͷ�����̼��֤��ú�к���̼Ԫ�غ���Ԫ�ء�
��������⡿ͨ��ʵ��֤��ú�к���̼Ԫ�غ���Ԫ�ء�
���������ϡ��١�����������ʹ���������Һ��ɫ�����Ϻ�ɫ��Ϊ��ɫ�����÷�Ӧ�Ļ�ѧ����ʽΪ��5SO2 + 2KMnO4 + 2H2O="==" K2SO4 + 2MnSO4 + 2 �������������ѧ����ʽ�����һ�����ʵĻ�ѧʽӡˢ����������Ʋ��仯ѧʽ�� ��
�ڡ���������Ͷ�����̼һ����Ҳ��ʹ����ʯ��ˮ����ǡ�������д�÷�Ӧ�Ļ�ѧ����ʽ�� ��
�����������ϣ���ͬѧ����ͬѧ�ֱ����ʵ�鷽������̽����
��1����ͬѧ��
������ʵ�顿��ͬѧ����������ͼ��ʾA��B����ʵ�飺

���۲�����A��ϡ���������Һ��ɫ��B�г���ʯ��ˮ����ǡ�
���ó����ۡ�úȼ�����ɶ�������Ͷ�����̼��֤��ú�к���̼Ԫ�غ���Ԫ�ء�
����Ϊ��ʵ�鷽���Ƿ�������������ɣ� ��
��2����ͬѧ��
������ʵ�顿��ͬѧ����������ͼ��ʾʵ�飨����װ����ͼ����ȥ����

Bװ�õ������� ��
���۲�����
�����B��C��D�����
���ó����ۡ�úȼ�����ɶ�������Ͷ�����̼��֤��ú�к���̼Ԫ�غ���Ԫ�ء�
���������ϡ���H2SO4 ��1�֣� �� SO2+Ca(OH)2===CaSO3��+H2O��1�֣�
��1������������Ϊ��������Ҳ��ʹ����ʯ��ˮ����ǣ���֤��ú�к���̼Ԫ�ء���1�֣�
��2�������������1�֣� B����Һ��ɫ��C����Һ��Ϊ�Ϻ�ɫ��D����Һ�����
��1������������Ϊ��������Ҳ��ʹ����ʯ��ˮ����ǣ���֤��ú�к���̼Ԫ�ء���1�֣�
��2�������������1�֣� B����Һ��ɫ��C����Һ��Ϊ�Ϻ�ɫ��D����Һ�����
������������������ϡ����ɷ�Ӧ�Ļ�ѧ����ʽ5SO2 + 2KMnO4 + 2H2O=K2SO4 + 2MnSO4 + 2 ��֪��
��Ӧǰ ��Ӧ��
��ԭ�� 5 3
��ԭ�� 20 12
��ԭ�� 2 2
��ԭ�� 2 2
��ԭ�� 4 0
���������غ㶨�ɣ���ѧ�仯ǰ��ԭ�ӵ����ࡢ��Ŀ���䣬���ж�������2��δ֪�����к���2����ԭ�ӡ�8����ԭ�Ӻ�4����ԭ�ӣ���1��δ֪�����к���1����ԭ�ӡ�4����ԭ�Ӻ�2����ԭ�ӣ����δ֪���ʵĻ�ѧʽΪH2SO4��
�ڸ������⣬����������Ͷ�����̼һ����Ҳ��ʹ����ʯ��ˮ����ǡ�����϶�����̼���������Ʒ�Ӧ�Ļ�ѧ����ʽ��֪������������������Ʒ�Ӧ�Ļ�ѧ����ʽΪSO2+Ca(OH)2=CaSO3��+H2O��
��1���������Ͽ�֪��Aʵ���е�ϡ���������Һ��ɫ��˵����Ӧ���ж�����������ɣ�����˵��ú�к���Ԫ�أ���Bʵ���еij���ʯ��ˮ����ǣ�˵����Ӧ���ж�����̼�������������ɣ�����˵��ú�п��ܺ���̼Ԫ�أ�Ҳ���ܺ�����Ԫ�أ��ʸ÷�����������
��2�����ڸ������Ͽ�֪������������ʹ���������Һ��ɫ�����Ϻ�ɫ��Ϊ��ɫ������Bװ�õ������Ǽ����������Ĵ��ڣ���Ũ���������Һ�����������Ӧ����ȥ��������ͬʱͨ�����������Һ�ֿɼ�����������ѱ����������ų������ó���ʯ��ˮ�������֤��������̼�Ĵ��ڣ��ʿɹ۲쵽��B��ϡ���������Һ��ɫ�� C�е�Ũ���������Һ��Ϊ�Ϻ�ɫ��D�г���ʯ��ˮ����ǡ�
������������֤��ʵ��̽����Ҫ������ʵ����ʻ�仯���ɣ����ݸ�����ʵ����Ʒ���������ʵ�顢������̽������ͨ���۲졢��¼�ͷ�����ʵ����������֤�����ʵ����ʻ�仯���ɵȡ�

��ϰ��ϵ�д�
�����Ŀ


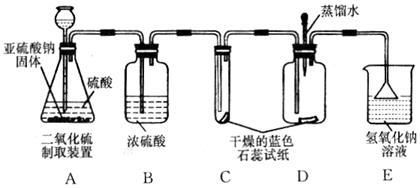
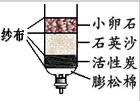

 ����ͬѧ��Ϊ���ز����Ȼ�þ����ɫ�������� ��
����ͬѧ��Ϊ���ز����Ȼ�þ����ɫ�������� ��