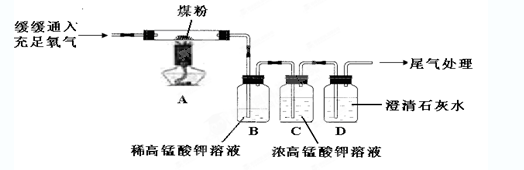
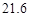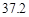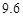��Ŀ����
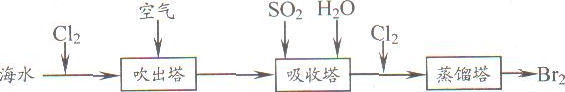
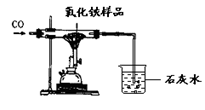
ij�������N��������H2��CO��CO2�е�һ�ֻ�������ɣ�Ϊ�˽�����������ijͬѧ���������װ�ý���ʵ�飨����NaOH��Һ������ȫ����CO2����

�±����ǶԿ��ܳ��ֵ�ʵ��������������ݴ��Ʋ�����N����ɽ����ȷ���ǣ�

�±����ǶԿ��ܳ��ֵ�ʵ��������������ݴ��Ʋ�����N����ɽ����ȷ���ǣ�
| | ���ܳ��ֵ�ʵ������ | ����N����� | ||
| X��Һ�� | Y�к�ɫ��ĩ | Z��Һ�� | ||
| A | ������ | ��ɹ����ĺ�ɫ | ������ | H2 |
| B | ������ | ��ɹ����ĺ�ɫ | ����� | CO��CO2 |
| C | ����� | ��ɹ����ĺ�ɫ | ������ | H2��CO2 |
| D | ����� | ��ɹ����ĺ�ɫ | ����� | H2��CO |
AC
���������X�г���ʯ��ˮ����ǣ�˵�������к��ж�����̼����������ǣ���˵�������в���������̼������X��Y�м�ʹ��������������Һ��������ԭ�������Ƿ��ж�����̼������Y�е����岻�ٺ��ж�����̼������Z�г���ʯ��ˮ����ǣ�˵���ж�����̼��������ԭ�����к���һ����̼����֮��˵��������û��һ����̼����Y�к�ɫ��ĩ��Ϊ�����ĺ�ɫ��˵���������л�ԭ�Ե����塣��ѡAC
�������������������������һ����̼�Ͷ�����̼�����ʣ��Լ���ط�Ӧ�������ǽ����Ĺؼ������ڲ���ֱ�Ӽ���ģ�Ҫ�����䷴Ӧ�IJ��

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ


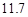 g NaCl��
g NaCl��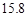 g NH4HCO3�������ϴ���Һ���������������Ϊ�� ��g��
g NH4HCO3�������ϴ���Һ���������������Ϊ�� ��g��