��Ŀ����
�����������ҹ�����������������Χ�����ɺ���ȫ�������Ž�һ�£���ͬ�������Ѽ���Ч���ش�
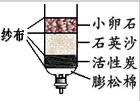
��1����Щ��ׯ���ȡ�õ���ˮ�����ü��ķ������������Ӳˮ������ˮ�������� ��
��2��ijͬѧ�����ǵ�ˮ�ü���ˮ������ͼ�����о����������������������õ��� ��
��3��Ϊ�˻��⺵�飬����������������ʵʩ�˹����꣬�����������˹������������ ������ţ�A����ʯ�� B������ C���ɱ�
��4����ϧˮ��Դ���������������һ����Լ��ˮ�������� ��

��1����Щ��ׯ���ȡ�õ���ˮ�����ü��ķ������������Ӳˮ������ˮ�������� ��
��2��ijͬѧ�����ǵ�ˮ�ü���ˮ������ͼ�����о����������������������õ��� ��
��3��Ϊ�˻��⺵�飬����������������ʵʩ�˹����꣬�����������˹������������ ������ţ�A����ʯ�� B������ C���ɱ�
��4����ϧˮ��Դ���������������һ����Լ��ˮ�������� ��
��1������ˮ����2������̿����3��C����4����ʱ�ر�ˮ��ͷ��
����������������е�֪ʶ���з�����Ӳˮ�к��еĽ϶�Ŀ����Ը�þ�������������ˮ������ɲ����Ը�þ������Ӷ��γɸ���������̿���������ԣ�������ɫ�غ���ζ���ɱ��������ȣ��������˹����꣬��Լ��ˮ���Դ�С�����𣮣�1��Ӳˮ�������ˮ����γɸ���������ˮ�����ˮ����γ���ĭ������ʹ�÷���ˮ����Ӳˮ����ˮ�����Ա����Ϊ������ˮ��
��2������̿���������ԣ����Ա����Ϊ������̿��
��3���ɱ��������ȣ���ʹ��Χ�¶Ƚ��ͣ���Ӧ�����˹����꣬���Ա����Ϊ��C��
��4������ˮ��ʱ�ر�ˮ��ͷ�Ϳɴﵽ��Լ��ˮ��Ŀ�ģ����Ա����Ϊ����ʱ�ر�ˮ��ͷ��
���������⿼����Ӳˮ����ˮ�ļ����Լ��������ʵ����ʣ���Ŀ��Ϊ�������������е�֪ʶ���м��ɣ�

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
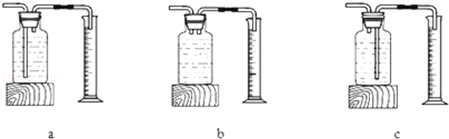
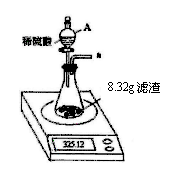

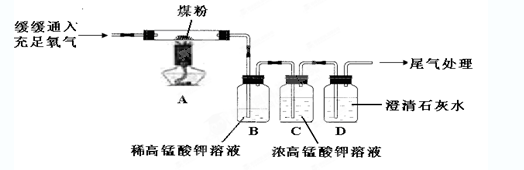
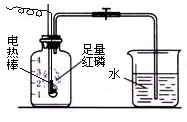

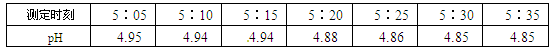
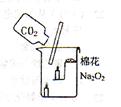

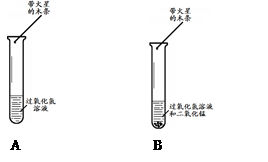

 ʱ��/s
ʱ��/s