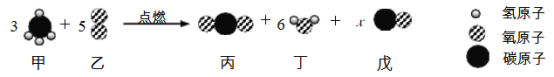��Ŀ����
����Ŀ��ͭԪ�ع㷺�����ڸ��������У���CuFeS2��Cu2(OH)2CO3��Cu-Zn�Ͻ�ȡ�
(1)����Cu����;________��(дһ��)
(2)д��CuFeS2��Cu��������������ʽ_________(���軯��)��
(3)��Ҫ�ⶨijCu-Zn�Ͻ���Cu����������ȡl3g�úϽ��ĩ���ձ���,��ϡHCl�����ε��룬ʵ���������±���ʾ��
ʵ���� | ��һ�� | �ڶ��� | ������ |
�����ϡHCl������/g | 100 | 100 | 100 |
��������������/g | 0.08 | 0.16 | 0.16 |
�ٷ����ϱ���ǡ����ȫ��Ӧ���ǵ�__________��ʵ�顣
�ڼ���:��l3g�Ͻ���Cu������______��(���ݻ�ѧ����ʽд�������ļ��㲽��)��
���𰸡�������(��������) ![]() �� 7.8g
�� 7.8g
��������
��1������ͭ�ļ۸��Ϊ���������Ҿ������õĵ����ԡ�����ͭ˿�����������ߡ��ʴ�Ϊ�������ߣ��𰸺������ɣ���
��2��CuFeS2��Cu����������ΪͭԪ�ص���������Է�������֮�ȣ�����![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
��3�����ڵ�����ʵ�鷴Ӧ����������������͵ڶ��η�Ӧ����������������ͬ����˵���ڶ���ʵ��ǡ����ȫ��Ӧ��
��:��úϽ���Zn������Ϊx
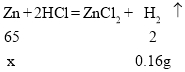
![]()
x=5.2g
��l3g�Ͻ���Cu������Ϊ: 1l3g-5.2g=7.8g
��:��13g�Ͻ���Cu������Ϊ7.8g��