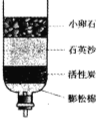
��Ŀ����
����Ŀ��(7��) ��������Ҫ�ɷ���̼��ơ�ij��ȤС��Ϊ�˲ⶨ��������CaCO3�ĺ�������ȡ15 g�����ǣ����飬�����ձ��У�Ȼ�������м���80 gijŨ�ȵ�ϡ���ᣬʹ֮��ַ�Ӧ���������г�CaCO3��������ɷֶ�������ˮ���Ҳ���ϡ���ᷴӦ��������ձ��еķ�Ӧʣ�����������m���뷴Ӧʱ�䣨t���Ĺ�ϵ��ͼ��ʾ������ˮ�����Ļӷ��������е���Ӧ���е�B��ʱ����������պ������˼�������һ�롣�Լ��㣨����������1λС��������������HCl����ˮ�γɵ���Һ����Ӧ��ˮ���μӷ�Ӧ��
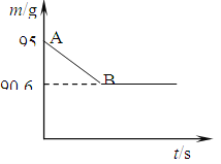
��1������CO2������Ϊ__________ g��
��2���ü�������CaCO3��������������3������ϡ���������μӷ�Ӧ��HCl��������
���𰸡���1��4.4g ��2��66.6%����3��7.3g��
��������
���������CaCO3+ 2HCl=CaCl2+ CO2��+H2O.���������غ㶨�ɿɵ�m(CO2)= 15 g��80g-90.64.4g����2�����輦�����к��еĴ���CaCO3����Ϊx�����ݷ���ʽ��CaCO3�� CO2��������ϵ��֪��100��:44=x:4.4�����x=10g�������ü�������CaCO3������������(10g��15 g)��100%=66.6%.��3���ɷ���ʽ��֪�����ʼ�Ĺ�ϵ��73:44=m(HCl):4.4.����m(HCl)=7.3g.�������ϡ���������μӷ�Ӧ��HCl������7.3g��

����Ŀ�����ձ�����μ���x��Һ����������Ӧ���������ɳ�������������������x��Һ��������ϵ������ͼ��ʾ�����߱�ʾ����


��� | �ձ��е����� | x��Һ |
�� | ͭп�Ͻ� | ϡHCl |
�� | ������ϡ���� | Na2CO3��Һ |
�� | ��������� | ϡHCl |
�� | H2SO4��CuSO4��Һ | NaOH��Һ |
A���٢� B���ڢۢ� C���٢ڢ� D���ۢ�