��Ŀ����
����Ŀ���������ʵ���ҳ�����һ���Լ���
��1�����������___��___���ɵ�(��д������)��
��2��������м�Ԫ�ص�����������__________(����ðٷ�����ʾ��������0.1%)��
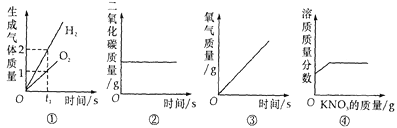
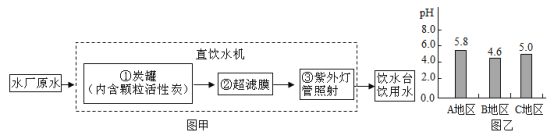
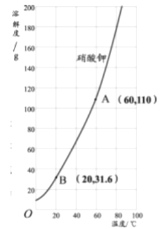
��3����ͼ������ص��ܽ�����ߣ���ͼ�����ܽ������ص��ܽ�����¶ȱ仯��������_________��60��ʱ110g���������100gˮ���γɵ���Һ��____(ѡ����͡������͡�)��Һ������ʱ��õ���Һ������20�棬��������ؾ����������___g��
(4)��ʽ����:��100g10%���������Һ����5%���������Һ����Ҫ��ˮ��������____?(ˮ���ܶ�Ϊ1.0g��mL-1)
���𰸡�K+;NO3-38.6%�����¶ȵ���������ص��ܽ��������78.4100mL
��������
��1��������ɼ����Ӻ���������ӹ��ɣ���2������Ԫ�ص�����������ʽ���㣻��3���������ص��ܽ������ͼ��������4������ϡ��ǰ�����ʵ��������������
��1�����������K+��NO3���ɵģ�
��2��������м�Ԫ�ص�����������![]() ��100%��38.6%��
��100%��38.6%��
��3��ͨ����������ص��ܽ�����ߣ������ܽ������ص��ܽ�����¶ȱ仯�������ǣ�����ص��ܽ�����¶ȱ仯Ӱ��ϴ�60��ʱ������ص��ܽ����110g������110g���������100gˮ���γɵ���Һ�DZ�����Һ������ʱ��õ���Һ������20�棬����ص��ܽ����31.6g��������������ؾ����������110g-31.6g=78.4g��
��4����100g 10%���������Һ����5%���������Һ����Ҫ��ˮ![]() -100g=100g�����Ϊ100g��1g/mL=100mL��
-100g=100g�����Ϊ100g��1g/mL=100mL��

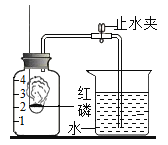
����Ŀ��Ϊ̽�������Թ�������(H2O2)�ֽ�Ĵ�Ч����ij�о�С��������ͼ��ʾ��ʵ�顣
ʵ��һ��
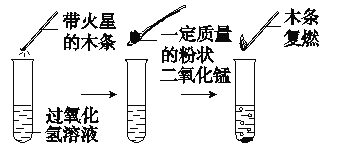
ʵ��������ݼ�¼��
1%����������Һ/mL | 50 | 50 | 50 |
����������̵�����/g | 0.1 | 0.2 | 0.4 |
����40 sĩ�õ����������/mL |
ʵ��һ��ͼ�е�ʵ���ܷ�֤�����������ǹ�������ֽⷴӦ�Ĵ�������˵�����ɣ�__________��
ʵ������ӱ������ƿ��Կ�������ʵ���Ŀ����____________��