��Ŀ����
����Ŀ��̼���ƹ㷺������ֽ����֯���Ƹ�ȹ�ҵ����һ����Ҫ�Ļ���ԭ�ϡ��ҹ���°����˽��Ƽ����ư���������������Ƽ��Ϊ̼���ƵĹ�ҵ�����������˾��ס�
���������ϣ������Ƽ����Ҫ��Ӧ��
��NaCl+NH3+CO2+H2O=NaHCO3��+NH4Cl
��2NaHCO3=Na2CO3+H2O+CO2��
��ʵ��̽����С���Դ������Ʊ���ʳ��ˮ����������ͼ1��ʾװ��ģ���Ʊ�̼�����ƣ������Ƶ�̼���ơ�
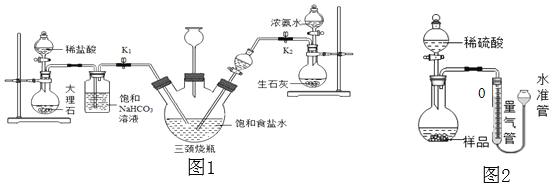
ʵ��������£�
�ٹر�K1����K2ͨ��NH3�������������ʣ������ȶ���K1ͨ��CO2��
�ڴ�������ƿ�ڳ��ֽ϶����ʱ���ر�K2ֹͣͨNH3��һ��ʱ��ر�K1ֹͣͨCO2��
�۽�������ƿ�ڵķ�Ӧ�������ˡ�ϴ�ӡ����¸���������ù������ڳ��������м��ȣ���¼ʣ�������������ش��������⣺
����ʱ��/min | t0 | t1 | t2 | t3 | t4 | t5 |
ʣ���������/g | δ��¼ | 15.3 | 13.7 | 11.9 | 10.6 | 10.6 |
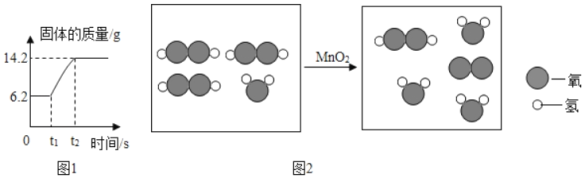
��1�������г��˺�����ɳ�Ȳ��������ʣ�������������MgCl2�ȡ���Ҫ��ȥ�����е�MgCl2���ɼ��������NaOH��Һ��д����Ӧ�Ļ�ѧ����ʽ______���ٹ��ˣ�Ȼ������Һ�м�������______���õ��Ȼ�����Һ��
��2������NaHCO3��Һ�������dz�ȥCO2�л��е�HCl����Ӧ�Ļ�ѧ����ʽΪ______��
��3��������ƿ�����ӵij���©������Ҫ������______����ͬѧ��ΪӦ���ڳ���©���ڷ���һ��պ����Һ������������______���ر�K2ֹͣͨNH3��Ҫ����ͨһ��ʱ��CO2����Ŀ����______��
��4������ʵ���¼������t2ʱNaHCO3����ķֽ��ʣ��ѷֽ��NaHCO3���������ǰԭNaHCO3�����ı�ֵ������д���������_____��
����չ���죩С�������ͼ2��ʵ��װ�òⶨij������Ʒ������������NaCl����̼���Ƶ�����������ȡһ��������Ʒ������ϡ���ᷴӦ��ͨ���ű���̼��������Һ�ⶨ����CO2�������������Ʒ��̼���Ƶ���������������֪���³�ѹ��CO2���ܶ���1.977g/L��
��1�������Ʒ����Ϊ5.3g����ʵ����ѡ�õ������ܹ������ʵ���______������ĸ����
A��500mLB��1000mLC��1500mLD��2000mL
��2����ʵ���в��̼������������ƫС�����п��ܵ�ԭ����______��
a��װ��©��
b����ȡ����ʱ�����ܵ�Һ�����ˮ�ܵ�Һ��
c����Ӧ��������ƿ����CO2����
d����ȡ����ʱ���Ӷ���
e��ϡ���������ƿռ�����
���𰸡�MgCl2+2NaOH=Mg(OH)2��+2NaCl ϡ���� NaHCO3+HCl=NaCl+H2O+CO2�� ƽ����ѹ ��ֹ�����ݳ���Ⱦ���� ʹ������ַ�Ӧ ��ԭ�������̼�����Ƶ�����Ϊx
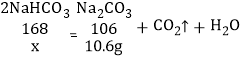
![]()
x=16.8g
��ȫ��Ӧʱʣ��������16.8g-10.6g=6.2g��
t2ʱ���������������16.8g-13.7g=3.1g��
t2ʱ������������ٵ�����Ϊ��ȫ��Ӧʱ��һ�룬��t2ʱNaHCO3����ķֽ���Ϊ50%�� C ab
��������
[ʵ��̽��]��1���Ȼ�þ���������Ʒ�Ӧ�����Ȼ��ƺ�������þ�������ʷ�Ӧ�Ļ�ѧ����ʽдΪ��MgCl2+2NaOH�TMg��OH��2��+2NaCl��
��ʱ����Һ�г����Ȼ������⣬�������������ƣ��ɼ������������Ὣ���к�Ϊ�Ȼ��ƣ�����ϡ���ᡣ
��2���Ȼ�����������ˮ�γ����ᣬ̼�����������ᷴӦ�����Ȼ��ơ�ˮ�Ͷ�����̼�����NaHCO3+HCl�TNaCl+H2O+CO2����
��3������©������������ͬ��������ƿ�����ӵij���©������Ҫ������ƽ����ѹ������ƽ����ѹ��
�ڳ���©���ڷ���һ��պ����Һ������������������ˮ���ᷴӦ���ܷ�ֹ�����ݳ���Ⱦ�����������ֹ�����ݳ���Ⱦ������
�ر�K2ֹͣͨNH3��Ҫ����ͨһ��ʱ��CO2����Ŀ�����γ����Ի���������ʹ������ַ�Ӧ������ʹ������ַ�Ӧ��
��4����ԭ�������̼�����Ƶ�����Ϊx
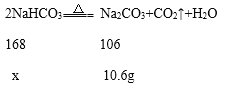
![]()
x=16.8g
��ȫ��Ӧʱʣ��������16.8g-10.6g=6.2g��
t2ʱ���������������16.8g-13.7g=3.1g��
t2ʱ������������ٵ�����Ϊ��ȫ��Ӧʱ��һ�룬��t2ʱNaHCO3����ķֽ���Ϊ50%��
[��չ����]��1�����5.3g��Ʒȫ��Ϊ̼���Ƶ�����������ݷ���ʽNa2CO3+2HCl�T2NaCl+H2O+CO2����̼�����������̼���������ϵ֪�����ɶ�����̼������Ϊ2.2g������V=![]() =
=![]() =1.1L=1100mL����ʵ����ѡ�õ������ܹ������ʵ���1500mL������C��
=1.1L=1100mL����ʵ����ѡ�õ������ܹ������ʵ���1500mL������C��
��2��a��װ��©������ռ����Ķ�����̼ƫ�٣���ɲⶨ���ƫС��
b����ȡ����ʱ�����ܵ�Һ�����ˮ�ܵ�Һ���������Ͳ�ڵ�ѹǿ�ϴ�����ռ��Ķ�����̼���ƫС�����²ⶨ���ƫС��
c����������Ͳ��Һ������������ƿ���ų��������������������Ͳ��Һ�������������Ϊ������̼�������������ݶ�����̼��������̼���Ƶ����������������ƿ������CO2������ʵ������Ӱ��
d����ȡ����ʱ���Ӷ�����ɶ�ȡ�Ķ�����̼���ƫ���²ⶨ���ƫ��
e��ϡ���������ƿռ�������ɶ�����̼���ƫ���²ⶨ���ƫ����ab��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ������ͼ����ʾԪ�صĻ��ϼ�����������ϵ��������ʾ��Ԫ�صIJ��ֹ�ϵͼ����:
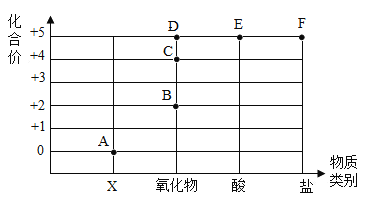
��1�� A���Ӧ�����������______��
��2��д��B�㡢E���ʾ���ʵĻ�ѧʽ��һ��Ϊ _________ ��_______ ��
��3��ij������Ļ�ѧʽΪKNO3���������ĵ���______(����ĸ)��
��4���������ʵ�_____________���������ʷֳɻ����ʹ����
��5����ɼ����Ȼ��غ��Ȼ�����ֹ��廯�ʵ�ʵ�鱨�档
ʵ�鲽�� | ʵ������ | ʵ����� |
_________ | _________ | _________ |
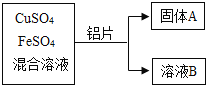
����Ŀ������м����Ҫ�ɷ��������÷���м��������������������ͼ��ʾ���������м�������ɷֲ����Ҳ��μӷ�Ӧ��
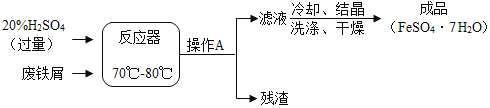
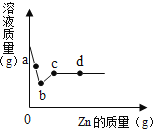
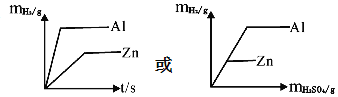
��1��������20%��H2SO4��Һ100�ˣ���Ҫ98%��Ũ������ٿˣ�____�������ȷ��0.1��
��2��ʵ���У�����AΪ______��
��3����ϴ��������FeSO47H2O����ʱ��һ�����������ˮϴ�ӣ����������������ܽ�ȱ��������ʾ����˵һ˵��������������______��
�¶�/�� | 10 | 20 | 30 | 40 | 50 | 60 |
�ܽ��/g | 20.3 | 26.3 | 30.8 | 40.1 | 48.4 | 52.4 |