��Ŀ����
����Ŀ�������ÿ�ѧ����ʶ����ķ�ʽ��ʶ����������

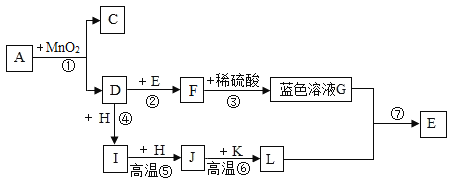
��1���ӷ���Ƕȣ���������______�����������������������������
��2�����۽Ƕȣ�����![]() ����ʾ��ԭ�ӣ���
����ʾ��ԭ�ӣ���![]() ����ʾ��ԭ�ӡ�
����ʾ��ԭ�ӡ�
������![]() ���ɱ�ʾ������______���ѧʽ����
���ɱ�ʾ������______���ѧʽ����
��ͬ��ͬѹ�£����������ȵ��ڷ�����Ŀ�ȡ��������������ɷֺ��Բ��ƣ���ͼ1�ɱ�ʾ������ģ�͵���______�����ţ���
��3���ӱ仯�Ƕȣ���ȼ�ŵ�ľ������ʢ��Һ̬�������ձ��ڣ��۲쵽��������______�������ձ�����һ��ʱ���Һ̬����ʣ��Լ![]() ���ʱ������Ҫ�ɷ���______��д��ѧʽ����
���ʱ������Ҫ�ɷ���______��д��ѧʽ����
��4����Ӧ�ýǶȣ����ÿ�����ȡ���ʵ�������ͼ2��ʾ��
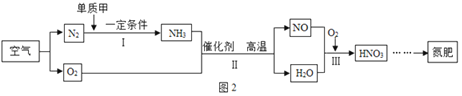
�٢��вμӷ�Ӧ�ĵ��ʼ�Ϊ______�������ƣ���
�ڱ��HNO3�е�Ԫ�صĻ��ϼ�______��
��д����Ӧ�Ļ�ѧ����ʽ______��
��5���ӻ����Ƕȣ����б���������ʩ��������______�����ţ���
A ��ʱ���������ƴ��ù�ҵ������ͽ������������ٵ����ﳾ
B ����ͨ���Ӹ��̴�ֱ���ŷŷ���
C �ᳫ���С������г�������̼�����з�ʽ
���𰸡������ N2 C ľ��Ϩ�� O2 ���� ![]() 3 4NH3+5O2
3 4NH3+5O2![]() 4NO+6H2O AC
4NO+6H2O AC
��������
��1���������ɵ����������ȶ���������ɵģ����ڻ����������
��2�����á�![]() ���ɱ�ʾ������һ�������ӣ�ÿ���������������ֵ�ԭ�ӹ��ɵģ���д�����ӵķ���ʱ���ڵ�Ԫ�ط������½�д��һ���������еĵ�ԭ�Ӹ���2���ɣ���N2������,N2��
���ɱ�ʾ������һ�������ӣ�ÿ���������������ֵ�ԭ�ӹ��ɵģ���д�����ӵķ���ʱ���ڵ�Ԫ�ط������½�д��һ���������еĵ�ԭ�Ӹ���2���ɣ���N2������,N2��
������Լռ���������![]() ������Լռ���������
������Լռ���������![]() ��ͬ��ͬѹ�£����������ȵ��ڷ�����Ŀ�ȣ���ͼ�п�����ģ���е������������ӵĸ�����ӦΪΪ4:1������C��
��ͬ��ͬѹ�£����������ȵ��ڷ�����Ŀ�ȣ���ͼ�п�����ģ���е������������ӵĸ�����ӦΪΪ4:1������C��
��3��Һ���ķе�ͣ���������������������֧��ȼ�գ����Խ�ȼ�ŵ�ľ������ʢ��Һ̬�������ձ��ڣ��۲쵽��������ȼ�ŵ�ľ��Ϩ�𣬹���ľ��Ϩ��
�����ձ�����һ��ʱ���Һ̬����ʣ��Լ![]() ���������Ҫ�ɷ�������������O2��
���������Ҫ�ɷ�������������O2��
��4���ٵ�����������һ�����������ɰ��������Ԣ٢��вμӷ�Ӧ�ĵ��ʼ�Ϊ����������������
�������У���Ԫ�صĻ��ϼ�Ϊ+1�ۣ���Ԫ�صĻ��ϼ�Ϊ-2�ۣ����ݻ������и�Ԫ�صĻ��ϼ۵Ĵ�����Ϊ�㣬��Ԫ����+5�ۣ���ʾΪ![]() ������
������![]() ��
��
�ۢ��з�Ӧ�ǰ����������ڴ������������·�Ӧ����һ��������ˮ���ʷ�Ӧ�Ļ�ѧ����ʽдΪ��4NH3+5O2![]() 4NO+6H2O��
4NO+6H2O��
��5��A ��ʱ���������ƴ��ù�ҵ������ͽ������������ٵ����ﳾ���Ը��ƿ���������ѡ����ȷ��
B ����ͨ���Ӹ��̴�ֱ���ŷŷ�������Ӹ����ϸ��ƿ���������ѡ�����
C���ᳫ���С������г�������̼�����з�ʽ���Լ��ٻ�ʯȼ�ϵ�ȼ�գ�������Ⱦ����ŷţ������ڸ��ƿ���������ѡ����ȷ������AC��

 ��У����ϵ�д�
��У����ϵ�д�����Ŀ��ˮ��һ����Ҫ������,���ճ�����ͺ�����ʵ�������Ų�����������á�
|
|
|
|
ͼ1 | ͼ2 | ͼ3 | ͼ4 |
(1)ˮ�ľ���
��ͼ1��ʾ��ˮ���ɳ�ȥˮ�е�ɫ�غ���ζ,����Ϊ���еĻ���̿����_____________�ԡ�
����ͼ1��ͼ2ʾ��ľ�ˮԭ����,�ܽ���ˮӲ�ȵľ�ˮ������ͼ______(����1����2)��
(2)ˮ����ɡ�
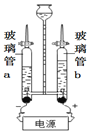
��ͼ3��ˮ�ĵ��װ��,��ֱͨ����Դһ��ʱ���,������b�ڲ�����������__________________,�÷�Ӧ�Ļ�ѧ����ʽΪ_____________________________��
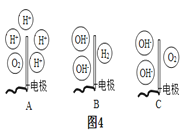
�ڵ��ˮ��ʵ����,�ڲ���������Ӻ��з�̪����������Һ,������ǿ�����ԡ���ʵ�������,�۲쵽��a�缫��������ҺѸ�ٱ��,��ô���Һ��_______(����������������������)�ԡ�����,����Һ��Ͼ��Ⱥ�����Һ��pH=7,��ͼ4���ܱ�ʾ���ʱ��b��ˮ�ڵ缫���������仯�õ���������_____(���Ӧѡ�����ĸ)��
(3)ˮ�ڻ�ѧʵ���о�����Ҫ���á�
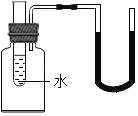
����˿���ڳ�ʪ�Ŀ�����(��ͼ5��ʾ),�ر�K,һ��ʱ���,�۲쵽������Һ������;��K,�۲쵽������Һ���½�,���ܿ������ݳ�������͵�����Һ���������½���ԭ��:_________________________��
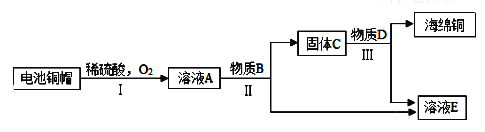
����Ŀ��̼���ƹ㷺������ֽ����֯���Ƹ�ȹ�ҵ����һ����Ҫ�Ļ���ԭ�ϡ��ҹ���°����˽��Ƽ����ư���������������Ƽ��Ϊ̼���ƵĹ�ҵ�����������˾��ס�
���������ϣ������Ƽ����Ҫ��Ӧ��
��NaCl+NH3+CO2+H2O=NaHCO3��+NH4Cl
��2NaHCO3=Na2CO3+H2O+CO2��
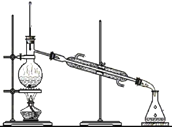
��ʵ��̽����С���Դ������Ʊ���ʳ��ˮ����������ͼ1��ʾװ��ģ���Ʊ�̼�����ƣ������Ƶ�̼���ơ�
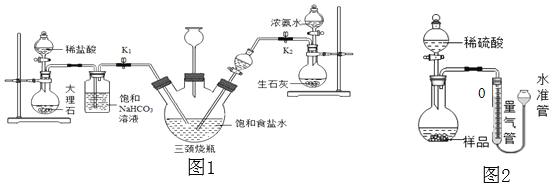
ʵ��������£�
�ٹر�K1����K2ͨ��NH3�������������ʣ������ȶ���K1ͨ��CO2��
�ڴ�������ƿ�ڳ��ֽ϶����ʱ���ر�K2ֹͣͨNH3��һ��ʱ��ر�K1ֹͣͨCO2��
�۽�������ƿ�ڵķ�Ӧ�������ˡ�ϴ�ӡ����¸���������ù������ڳ��������м��ȣ���¼ʣ�������������ش��������⣺
����ʱ��/min | t0 | t1 | t2 | t3 | t4 | t5 |
ʣ���������/g | δ��¼ | 15.3 | 13.7 | 11.9 | 10.6 | 10.6 |
��1�������г��˺�����ɳ�Ȳ��������ʣ�������������MgCl2�ȡ���Ҫ��ȥ�����е�MgCl2���ɼ��������NaOH��Һ��д����Ӧ�Ļ�ѧ����ʽ______���ٹ��ˣ�Ȼ������Һ�м�������______���õ��Ȼ�����Һ��
��2������NaHCO3��Һ�������dz�ȥCO2�л��е�HCl����Ӧ�Ļ�ѧ����ʽΪ______��
��3��������ƿ�����ӵij���©������Ҫ������______����ͬѧ��ΪӦ���ڳ���©���ڷ���һ��պ����Һ������������______���ر�K2ֹͣͨNH3��Ҫ����ͨһ��ʱ��CO2����Ŀ����______��
��4������ʵ���¼������t2ʱNaHCO3����ķֽ��ʣ��ѷֽ��NaHCO3���������ǰԭNaHCO3�����ı�ֵ������д���������_____��
����չ���죩С�������ͼ2��ʵ��װ�òⶨij������Ʒ������������NaCl����̼���Ƶ�����������ȡһ��������Ʒ������ϡ���ᷴӦ��ͨ���ű���̼��������Һ�ⶨ����CO2�������������Ʒ��̼���Ƶ���������������֪���³�ѹ��CO2���ܶ���1.977g/L��
��1�������Ʒ����Ϊ5.3g����ʵ����ѡ�õ������ܹ������ʵ���______������ĸ����
A��500mLB��1000mLC��1500mLD��2000mL
��2����ʵ���в��̼������������ƫС�����п��ܵ�ԭ����______��
a��װ��©��
b����ȡ����ʱ�����ܵ�Һ�����ˮ�ܵ�Һ��
c����Ӧ��������ƿ����CO2����
d����ȡ����ʱ���Ӷ���
e��ϡ���������ƿռ�����