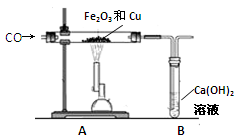
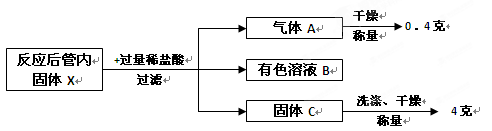
��Ŀ����
�����ǵ���Ҫ�ɷ���̼��ƣ�ij��ȤС��Ϊ�˲ⶨ��������CaCO3�ĺ�������ȡ15g�����ǣ����飬�����ձ��У�Ȼ�������м���80gijŨ�ȵ�ϡ���ᣬʹ֮��ַ�Ӧ���������г�CaCO3��������ɷֶ�������ˮ���Ҳ���ϡ���ᷴӦ��������ձ��еķ�Ӧʣ�����������m���뷴Ӧʱ�䣨t���Ĺ�ϵ��ͼ��ʾ������ˮ�����Ļӷ��������е���Ӧ���е�B��ʱ����������պ������˼�������һ�룬�Լ��㣨����������1λС����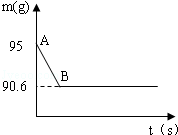
��1������CO2������Ϊ g��
��2���ü�������CaCO3������������
��3������ϡ���������ʵ�����������
��1��4.4g��2��66.7%��3��18.3%
����

��ϰ��ϵ�д�
�����Ŀ
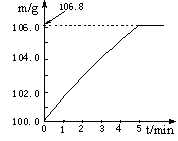
��3�֣�Ϊ�˲ⶨij��Ʒ��̼�ᱵ��������������������ʵ�顣ȡһ��������Ʒ��400g������������Ϊ10%��ϡ��������ձ��С��ڻ�ѧ��Ӧ�����ж��ձ������е�ʣ�����������γ�������¼���±��������跴Ӧ���ٽ��У����ʲ�����ˮ��Ҳ����ϡ���ᷴӦ���ձ�������Ϊ25��4g��
| ��Ӧʱ�� | t1 | t2 | t3 | t4 | t5 |
| �ձ���ҩƷ����/g | 516��6 | 507��8 | m | 503��4 | 503��4 |
����㣺����Ӧ����ʽΪ��BaCO3 + 2HCl = BaCl2 + H2O + CO2����
��1�����еġ�m��Ϊ��
��2���ò�Ʒ��̼�ᱵ������������
��3������Ʒ��̼�ᱵ��ϡ����ǡ����ȫ��Ӧʱ��������Һ�����ʵ�����������
(6��)Ϊ�ⶨCuһZn�Ͻ����ɣ�С��ͬѧ���øúϽ��ĩ��ϡ���ᷴӦ������������ʵ�飬�������ʵ�����ݼ�¼���£�
| | ��һ�� | �ڶ��� | ������ |
| ��ȡ�Ͻ������/g | 1O | 10 | 20 |
| �������������/g | 50 | 80 | 50 |
| ��������������/g | 0��2 | 0��2 | 0��2 |
��1�����ϱ����ݷ���������ȡ�Ͻ�������ϡ�����������Ϊ ʱ�������Ͻ��е�п��ϡ����ǡ����ȫ��Ӧ��
��2���úϽ���п������Ϊ���ٿ�?(����������һλС��)
��3�����úϽ��е�п��ϡ����ǡ����ȫ��Ӧʱ������Һ�����ʵ���������Ϊ����?(��������ȷ��0��1��)
 Na2SO4+2H2O+2NH3���� 2NH3+H2SO4=(NH4)2SO4��
Na2SO4+2H2O+2NH3���� 2NH3+H2SO4=(NH4)2SO4��