��Ŀ����
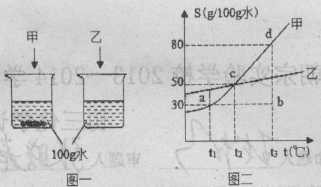
����Ŀ��(6��)����ʱ��ͬѧ�Dz����������ַ����ⶨij�Ȼ�����Һ����������������
��1������ѧ��������һ�����Ȼ�����Һ�м���������������Һ���õ�2.87g�Ȼ������壬����Ȼ�����Һ���Ȼ��Ƶ�����Ϊ���٣������ݻ�ѧ����ʽ��ʽ���㣩
�����ʵ��ⶨ������Һ��������������Ϊ10%��
��2��������������ȡһ��������Һ��������������ʵ���������£�
�������������g�� | 25.0 |
������+ʳ����Һ��g�� | 45.0 |
������+ʳ�ξ��壨g�� | 27.4 |
���ݴ��� | ��Һ��������������Ϊ |
����ѧ�����ⶨ���ȷ�������������ⶨ�����������ԭ����
A������ʱδ�ò��������� B����ȡ�Ȼ�����Һ�����ϴ�
C������ʱ������������ʱ��ֹͣ���� D��ʵ���δ���������ϵİ�ɫ��������������
���𰸡� ��1��1.17g����2��12 %��C
��������
�����������1���⣺�������Ȼ�������Ϊx
NaCl+AgNO3��AgCl�� +NaNO3
58.5 143.5
X 2.87g
![]()
x=1.17g
�𣺸��Ȼ�����Һ���Ȼ��Ƶ�����Ϊ1.17g��
��2����Һ��������������Ϊ��![]() 12%����ѧ�����ⶨ���ȷ�������������ⶨ�����������ԭ�������ʵ���������ƫ��ΪC����ʱ������������ʱ��ֹͣ������������ʵ��������ࡣ
12%����ѧ�����ⶨ���ȷ�������������ⶨ�����������ԭ�������ʵ���������ƫ��ΪC����ʱ������������ʱ��ֹͣ������������ʵ��������ࡣ

 ��ʦ�㲦��ϵ�д�
��ʦ�㲦��ϵ�д�����Ŀ��Ҫ��ȥ���и��������е��������ʣ���ѡ�Լ��ͷ��������е��ǣ� ��
ѡ�� | ���� | ���� | �Լ������� |
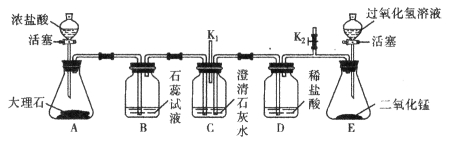
A | O2 | ˮ���� | ͨ��ŨH2SO4ϴ�� |
B | MnO2 | ̼�� | �ڿ��������� |
C | KCl��Һ | BaCl2 | ����������Na2SO4��Һ������ |
D | FeSO4��Һ | CuSO4 | ����������м����ַ�Ӧ����� |
A��A B��B C��C D��D