��Ŀ����
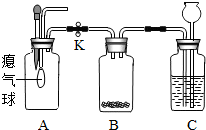
 ��ͼΪʵ�����ü��ȸ�����صķ�����ȡ������װ��ͼ���ش����⣺
��ͼΪʵ�����ü��ȸ�����صķ�����ȡ������װ��ͼ���ش����⣺��1��ָ���������������
a
e
��2��ͼ���м������Դ��������
��
��
��3��д��ͼ�з�����Ӧ�Ļ�ѧ����ʽ��
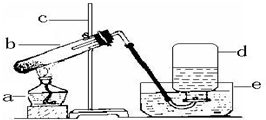
��4��ijͬѧ��������ʵ�鲽�����ʵ�飺�ٰ�Ҫ�����Ӻ������������Թ���װ������ĩ���̶�������̨�ϣ��۽�����ƿװ��ˮ����ˮ���У��ܼ����Թ��ռ��������ݽ���ʱ�����õ�ñ����ƾ��ƣ����Թ���ȴ�����Ƴ�ˮ�ۣ������������ͬѧ��ʵ����ȱ�ٵĹؼ�������
����
���㣺��������ȡװ��,�������ռ�����,��ȡ�����IJ��������ע���,��д��ѧ����ʽ�����ֱ���ʽ�����뷽��ʽ
ר�⣺���������ʵ�����Ʒ������顢�����뾻��
��������1����������ͼ����;ȥ�������
��2�����ڹ̶��Թ�ʱ���Թܿ�Ҫ����������б�������Թ��ڵĵ���������¶����Ƥ������ȥ�������
��3�����ü��ȸ�����صķ�����ȡ������������������ͬʱ����������غͶ�����������ȥ�������
��4������ȡ����ʱ����Ҫ�����Ӻ�������Ҫ�ȼ��װ�õ������ԣ�����ʱ��Ӧ�Ȱѵ����Ƴ�ˮ�棬��Ϩ��ƾ���ȥ�������
��2�����ڹ̶��Թ�ʱ���Թܿ�Ҫ����������б�������Թ��ڵĵ���������¶����Ƥ������ȥ�������
��3�����ü��ȸ�����صķ�����ȡ������������������ͬʱ����������غͶ�����������ȥ�������
��4������ȡ����ʱ����Ҫ�����Ӻ�������Ҫ�ȼ��װ�õ������ԣ�����ʱ��Ӧ�Ȱѵ����Ƴ�ˮ�棬��Ϩ��ƾ���ȥ�������
����⣺��1����������ͼ����;��֪aΪ�ƾ��ƣ�bΪ�Թܣ�cΪ����̨��dΪ����ƿ��eΪˮ�ۣ��ʴ�Ϊ���ƾ��� �Թ� ����̨ ����ƿ ˮ�ۣ�
��2���ڹ̶��Թ�ʱ���Թܿ�Ҫ����������б��������Ϊ���ڼ��ȵĹ�����ҩƷ��������к��е�ʪ��ˮ�ͻ���ˮ��������ʽ��˳�������������ܣ����������¶Ƚϵ͵��Թܿ�ʱ���ֻ�������ˮ�飬����Թܿ����ϣ���ʱˮ�ͻᵹ�����¶Ⱥܸߵ��Թܵײ����Ӷ������Թܵײ��������ը�ѣ���ȡ����ʱ��Ϊ���ž��Թ��ڵĿ����������Թ��ڵĵ���������¶����Ƥ�����ɣ��ʴ�Ϊ���ڹ̶��Թ�ʱ���Թܿ�Ҫ����������б �����Թ��ڵĵ���������¶����Ƥ�����ɣ�
��3���ü��ȸ�����صķ�����ȡ������������������ͬʱ����������غͶ����������ɣ����Ի�ѧ����ʽΪ��2KMnO4
K2MnO4+MnO2+O2�����ʴ�Ϊ��2KMnO4
K2MnO4+MnO2+O2����
��4����ȡ����ʱ����Ҫ�����Ӻ�������Ҫ�ȼ��װ�õ������ԣ���������Բ��ã����ռ���������ȡ�����壬���ҩƷ���˷ѣ��ݽ���ʱ�������Ϩ��ƾ��ƣ��ٰѵ����Ƴ�ˮ�����������Թܵ��¶�Խ��Խ�ͣ��Թ��ڵ����������Խ��ԽС���ڲ�����ѹ��С�����ⲿ����ѹ�������£�ˮ���ڵ�ˮ�ͻᱻ˳�ŵ���ѹ���Թ��ڣ�����ʹ�Թ��������Ȳ����Ӷ�ը�ѣ��ʴ�Ϊ�����װ�õ������� ����ʱ��Ӧ�Ȱѵ����Ƴ�ˮ�棬��Ϩ��ƾ��ƣ�
��2���ڹ̶��Թ�ʱ���Թܿ�Ҫ����������б��������Ϊ���ڼ��ȵĹ�����ҩƷ��������к��е�ʪ��ˮ�ͻ���ˮ��������ʽ��˳�������������ܣ����������¶Ƚϵ͵��Թܿ�ʱ���ֻ�������ˮ�飬����Թܿ����ϣ���ʱˮ�ͻᵹ�����¶Ⱥܸߵ��Թܵײ����Ӷ������Թܵײ��������ը�ѣ���ȡ����ʱ��Ϊ���ž��Թ��ڵĿ����������Թ��ڵĵ���������¶����Ƥ�����ɣ��ʴ�Ϊ���ڹ̶��Թ�ʱ���Թܿ�Ҫ����������б �����Թ��ڵĵ���������¶����Ƥ�����ɣ�
��3���ü��ȸ�����صķ�����ȡ������������������ͬʱ����������غͶ����������ɣ����Ի�ѧ����ʽΪ��2KMnO4
| ||
| ||
��4����ȡ����ʱ����Ҫ�����Ӻ�������Ҫ�ȼ��װ�õ������ԣ���������Բ��ã����ռ���������ȡ�����壬���ҩƷ���˷ѣ��ݽ���ʱ�������Ϩ��ƾ��ƣ��ٰѵ����Ƴ�ˮ�����������Թܵ��¶�Խ��Խ�ͣ��Թ��ڵ����������Խ��ԽС���ڲ�����ѹ��С�����ⲿ����ѹ�������£�ˮ���ڵ�ˮ�ͻᱻ˳�ŵ���ѹ���Թ��ڣ�����ʹ�Թ��������Ȳ����Ӷ�ը�ѣ��ʴ�Ϊ�����װ�õ������� ����ʱ��Ӧ�Ȱѵ����Ƴ�ˮ�棬��Ϩ��ƾ��ƣ�
��������������Ҫ���������������ơ��������ȡװ�ã�ͬʱҲ�����˻�ѧ����ʽ����д���ۺ��ԱȽ�ǿ�����������п�����Ҫ����֮һ����Ҫ������ʵ�����У�

��ϰ��ϵ�д�
 ��ɢ˼ά�¿���ϵ�д�
��ɢ˼ά�¿���ϵ�д�
�����Ŀ
���������û�ѧ������������Ĺ����У�������һϵ�еĿ�ѧ������Ϊָ���������������Ӧ�Ŀ�ѧ�۲�һ�µ��ǣ�������
| A����ѧ���������ߵ����ʺϳɷḻ��ʵ������ʣ����������ʵ�ת���� |
| B�����û�ѧ��Ӧ�������������硢ȡů����������������������ת���� |
| C��̽Ѱֱ�Ӱ�ˮ����͵ķ���������Ԫ�ص��غ�� |
| D���ڻ��������н�ԭ�Ͼ�����ȫ��ת��Ϊ��Ʒ����������ɫ��ѧ���� |
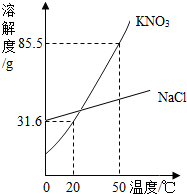 ijʵ����������һ����������������KNO3��Һ����ʵ����ֻ�к�����NaCl��KNO3����ҩƷ��
ijʵ����������һ����������������KNO3��Һ����ʵ����ֻ�к�����NaCl��KNO3����ҩƷ��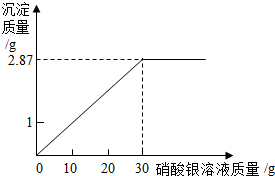 СǿΪ�ⶨij�Ȼ�����Һ�����ʵ�������������������ʵ�飺���ձ��м���10g���Ȼ�����Һ��Ȼ�������еμ���������Һ���μ���������Һ�����������������������ϵ��ͼ��ʾ����֪��NaCl+AgNO3�TNaNO3+AgCl����
СǿΪ�ⶨij�Ȼ�����Һ�����ʵ�������������������ʵ�飺���ձ��м���10g���Ȼ�����Һ��Ȼ�������еμ���������Һ���μ���������Һ�����������������������ϵ��ͼ��ʾ����֪��NaCl+AgNO3�TNaNO3+AgCl����