��Ŀ����
����Ŀ��ͬѧ��ͨ�����鵱��ȼ�ϵ���Դ��ʹ���������ȼ������һ������ʶ���������Ǽ���ij���ȼ��ʹ�ð�ȫ�dz���Ҫ��һ��С�ľͻ����Σ�ա�
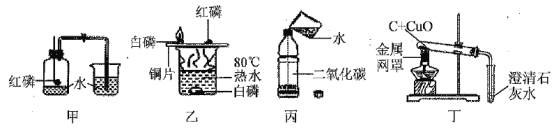
��1��Ŀǰ����ȼ����Ϊ��Ȼ������Ȼ������Ҫ�ɷ��Ǽ��飬����ȫȼ�յĻ�ѧ����ʽ��______��С���ڻ�ѧ����ѧ������ȼ�ջᷢ��_________���棬����������������ʱ��ȼ�������ʻ�ɫ�������кڡ������������ѧ֪ʶ�����е���������ָ�����������������______��
��2�������й���ȫȼ�պͲ���ȫȼ�յ�˵���У�����ȷ����____��
A ȼ��ȼ��ʱð��������Ϊ����ȫȼ��
B ��̼ȼ�ϵIJ���ȫȼ�ջ�����һ����̼���ж��������Ⱦ����
C ��ͬ������̼������ȫȼ�պͲ���ȫȼ��ʱ���ͷŵ�����һ����
D �ճ������е�ȼ����ȫȼ�նԽ�Լ��Դ���������������Ż���������
��3�����극��Ҫ��ʱ�ر���Ȼ����Ŀ��أ������ԭ����_________��С�����и��ܵ��������еĻ�ѧ���Ӵ˶Ի�ѧ������Ũ�����Ȥ��
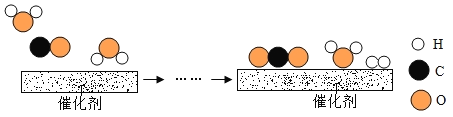
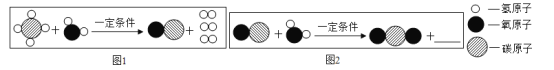
���𰸡�CH4++2O2![]() CO2+2H2O ��ɫ ���ڽ���ڣ�ʹ�������������ӣ�����ڽ����ڣ�ʹȼ�Ͻ��������٣� C �����ȼ��
CO2+2H2O ��ɫ ���ڽ���ڣ�ʹ�������������ӣ�����ڽ����ڣ�ʹȼ�Ͻ��������٣� C �����ȼ��
��������
��1������ȼ�������˶�����̼��ˮ����Ӧ�ķ���ʽ�ǣ�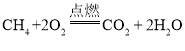 �� ����ȼ�ջᷢ����ɫ���棻������ֻ���ʻ�ɫ�������кڣ�˵��ȼ�ղ���֣���Ҫ���ڽ���ڣ�ʹ�������������ӣ�����ڽ����ڣ�ʹȼ�Ͻ��������٣���
�� ����ȼ�ջᷢ����ɫ���棻������ֻ���ʻ�ɫ�������кڣ�˵��ȼ�ղ���֣���Ҫ���ڽ���ڣ�ʹ�������������ӣ�����ڽ����ڣ�ʹȼ�Ͻ��������٣���
��2����ͬ������̼������ȫȼ�պͲ���ȫȼ��ʱ����ȫȼ���ͷŵ������࣬��ѡC��
��3�����ԭ����Ҫ�У����������������¶ȵ��Ż�����¡������ȼ����극��Ҫ��ʱ�ر���Ȼ����Ŀ��أ������ԭ���������ȼ�

����Ŀ�����л�Ͼ��ȵ�п����ĩ��ij��ѧ��ȤС��Ϊ�˲ⶨ����ɣ�ȡ30g��Ʒ���ձ��У������з�3�μ�����������������ͬ��ϡ���ᣬʹ֮��ַ�Ӧ��ÿ������ϡ�����������ʣ������������¼���±���
����ϡ�����������g�� | ��ַ�Ӧ��ʣ������������g�� | |
��1�� | 20 | 23.5 |
��2�� | 40 | 13.5 |
��3�� | 10 | m |
�Իش��������⣺
��1������������m��ֵΪ_____��
��2���ý�����ĩ��п�����������Ƕ���_____��
��3������ϡ��������������������Ƕ���_____��