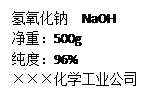��Ŀ����
��6�֣�����ͬѧ��ⶨ���������������������������������£���ش��������⡣
��1������ϡ���ᡣ
������200g 19.6%��ϡ���ᣬ��Ҫ98%��Ũ���������� ��
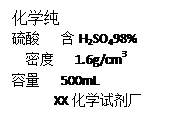
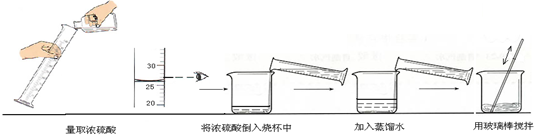
����ͼʵ������г��ֲ����Ĵ����� ��
(2)���������ĩ����ϡ�����ܽ⣬��Ӧ�Ļ�ѧ����ʽ�� ��
(3)ȡ20g�ij������ĩǡ����l50g��19.6����ϡ������ȫ��Ӧ�������������������(x)�ı���ʽΪ ��
(4)�˳�����������������������Ϊ ��
(5)��Ӧ��������Һ�м���34gˮ����������Һ���ʵ�����������
��1������ϡ���ᡣ
������200g 19.6%��ϡ���ᣬ��Ҫ98%��Ũ���������� ��
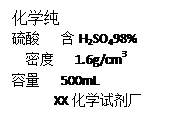
����ͼʵ������г��ֲ����Ĵ����� ��

(2)���������ĩ����ϡ�����ܽ⣬��Ӧ�Ļ�ѧ����ʽ�� ��
(3)ȡ20g�ij������ĩǡ����l50g��19.6����ϡ������ȫ��Ӧ�������������������(x)�ı���ʽΪ ��
(4)�˳�����������������������Ϊ ��
(5)��Ӧ��������Һ�м���34gˮ����������Һ���ʵ�����������
��1���� 26mL �ڶ����ķ�ʽ����Ũ�����ˮ�����˳��ߵ���
��2��Fe2O3+3H2SO4=Fe2��SO4��3+3H2O
��3��294/400=29.4g/x
��4��80%
��5��20%
��2��Fe2O3+3H2SO4=Fe2��SO4��3+3H2O
��3��294/400=29.4g/x
��4��80%
��5��20%
��1������Ҫ98%��Ũ����������=200g��19.6%��98%��1.6g/cm3=25mL��
�ʴ�Ϊ��25mL��������Ͳ��ȡҺ�����ʱ����Ҫ�밼Һ�����ʹ�����ˮƽ��ϡ��Ũ����ʱҪ�ȼ�ˮ��Ȼ��Ũ����ע��ˮ�У����Դ�Ϊ�������ķ�ʽ����Ũ�����ˮ�����˳��ߵ�
��2�����������Ҫ�ɷ�Ϊ���������������������������ᷴӦ������������ˮ���ʴ�Ϊ��Fe2O3+3H2SO4=Fe2��SO4��3+3H2O��
��3������������������x
Fe2O3+3H2SO4=Fe2��SO4��3+3H2O
294 400
150g��19.6% x
�ʴ�Ϊ��
294��400=50g��19.6%��x��
��4����������������Ϊy
Fe2O3+3H2SO4=Fe2��SO4��3+3H2O
160 294
Y 150g��19.6%
160��294= Y��150g��19.6%
y=16g
��������Ʒ������������������Ϊ��16g/20g��100%=80%��
�ʴ�Ϊ��80%��
��5����Ӧ��������Һ�м���34gˮ��������������Һ�����ʵ�����������20%��
�ʴ�Ϊ��20%
�ʴ�Ϊ��25mL��������Ͳ��ȡҺ�����ʱ����Ҫ�밼Һ�����ʹ�����ˮƽ��ϡ��Ũ����ʱҪ�ȼ�ˮ��Ȼ��Ũ����ע��ˮ�У����Դ�Ϊ�������ķ�ʽ����Ũ�����ˮ�����˳��ߵ�
��2�����������Ҫ�ɷ�Ϊ���������������������������ᷴӦ������������ˮ���ʴ�Ϊ��Fe2O3+3H2SO4=Fe2��SO4��3+3H2O��
��3������������������x
Fe2O3+3H2SO4=Fe2��SO4��3+3H2O
294 400
150g��19.6% x
�ʴ�Ϊ��
294��400=50g��19.6%��x��
��4����������������Ϊy
Fe2O3+3H2SO4=Fe2��SO4��3+3H2O
160 294
Y 150g��19.6%
160��294= Y��150g��19.6%
y=16g
��������Ʒ������������������Ϊ��16g/20g��100%=80%��
�ʴ�Ϊ��80%��
��5����Ӧ��������Һ�м���34gˮ��������������Һ�����ʵ�����������20%��
�ʴ�Ϊ��20%

��ϰ��ϵ�д�
 ������������Ӧ����ϵ�д�
������������Ӧ����ϵ�д� ͬ����չ�Ķ�ϵ�д�
ͬ����չ�Ķ�ϵ�д�
�����Ŀ
 2CO2��+ 4H2O + 3N2�� ������ȫȼ��30 tƫ�����»�������ٶ�ˮ��
2CO2��+ 4H2O + 3N2�� ������ȫȼ��30 tƫ�����»�������ٶ�ˮ��