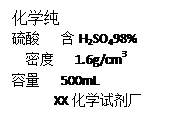��Ŀ����
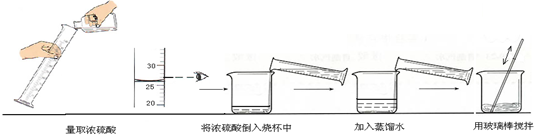
ij������������15%������������Һϴ��ϴ��һ����ʯ�Ͳ�Ʒ�еIJ������ᣬ����������������Һ40�ˣ�ϴ�Ӻ����Һ�����ԡ�
��1����������������������Һ480�ˣ�������ͼ��ǩ��ʾ��������������Ϊ ��
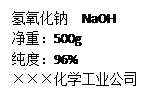
��2��������Ӧ�Ļ�ѧ����ʽ ��
��3������һ����ʯ�Ͳ�Ʒ�в�������������X���ı���ʽΪ ��
��4��������ͬ��������������������������Һȥǡ���к�һ����ʯ�Ͳ�Ʒ�еIJ������ᣬ����������������Һ����Ϊ ��
��5���к�ͬ�����������ᣬ���������ơ��������ص�������Ϊ ��
��1����������������������Һ480�ˣ�������ͼ��ǩ��ʾ��������������Ϊ ��
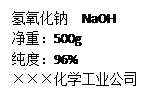
��2��������Ӧ�Ļ�ѧ����ʽ ��
��3������һ����ʯ�Ͳ�Ʒ�в�������������X���ı���ʽΪ ��
��4��������ͬ��������������������������Һȥǡ���к�һ����ʯ�Ͳ�Ʒ�еIJ������ᣬ����������������Һ����Ϊ ��
��5���к�ͬ�����������ᣬ���������ơ��������ص�������Ϊ ��
��1��75g
��2��2NaOH+H2SO4 = Na2SO4+2H2O
��3��80/98=40g��15%/x ���������ɣ�
��4��56g
��5��5��7
��2��2NaOH+H2SO4 = Na2SO4+2H2O
��3��80/98=40g��15%/x ���������ɣ�
��4��56g
��5��5��7
����1������15%����������Һ480g��Һ��Ҫ����������96%���������Ƶ�����=480g��15%��96%=75g��
��2���������������ᷴӦ���������ƺ�ˮ����Ӧ�Ļ�ѧ����ʽΪ2NaOH+H2SO4=Na2SO4+2H2O��
��3�����ݷ�Ӧ�Ļ�ѧ����ʽ��һ����ʯ�Ͳ�Ʒ�в�������������X���������������ƵĹ�ϵΪ
H2SO4+2NaOH=Na2SO4+2H2O
98 80
x 40g��15%
80 98 =40g��15% xx=7.35g
��4��������������������Һ����Ϊy
H2SO4+2KOH=K2SO4+2H2O
98 112
7.35g y��15%
98 7.35g ="112" y��15%
y=56g
��5���к�ͬ�����������ᣬ���������ơ��������ص�������=��40g��15%������56g��15%��=5��7
�ʴ�Ϊ��
��1��75��
��2��H2SO4+2NaOH=Na2SO4+2H2O��
��3��80 98 =40g��15% x ��
��4��56��
��5��5��7��
��2���������������ᷴӦ���������ƺ�ˮ����Ӧ�Ļ�ѧ����ʽΪ2NaOH+H2SO4=Na2SO4+2H2O��
��3�����ݷ�Ӧ�Ļ�ѧ����ʽ��һ����ʯ�Ͳ�Ʒ�в�������������X���������������ƵĹ�ϵΪ
H2SO4+2NaOH=Na2SO4+2H2O
98 80
x 40g��15%
80 98 =40g��15% xx=7.35g
��4��������������������Һ����Ϊy
H2SO4+2KOH=K2SO4+2H2O
98 112
7.35g y��15%
98 7.35g ="112" y��15%
y=56g
��5���к�ͬ�����������ᣬ���������ơ��������ص�������=��40g��15%������56g��15%��=5��7
�ʴ�Ϊ��
��1��75��
��2��H2SO4+2NaOH=Na2SO4+2H2O��
��3��80 98 =40g��15% x ��
��4��56��
��5��5��7��

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ