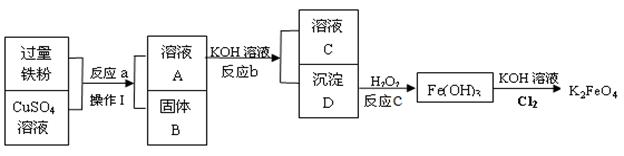
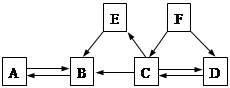��Ŀ����
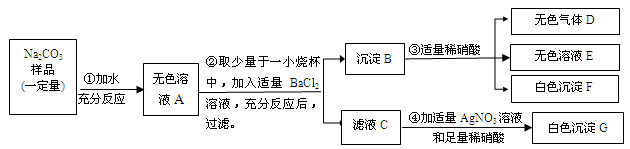
��7 �֣��������ε�����ʵ���ijʵ��С�����Է�Һ�ɷֽ����о��� ����ʵ������ҩƷ�� ���dz��������÷�Һ�п��ܺ��� CaCl2�� NaCl��Na2SO4��Na2CO3�е����ֻ���֡�Ϊȷ����Һ�ɷ֣����ǰ���ͼ��ʾ����ʵ�飬���ֵ�������ͼ������������������з����ķ�Ӧ��ǡ����ȫ���У��� �Ը���ʵ����̺ͷ�����������д���¿հף�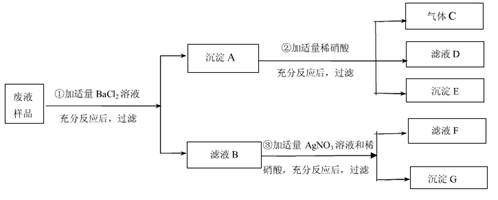
��1�������£����� C ˮ��Һ�� pH________7������ڡ�����С�ڡ����ڡ�֮һ�� ������ E �� ��д��ѧʽ�� ��
��2����Һ��һ�����е������� ��д��ѧʽ�� ��
��3����Һ F ��һ�����е��������� ��д���ӷ��ţ� ��
��4��������з�����Ӧ�Ļ�ѧ����ʽΪ ��
��5������������ʵ������Һ��Ʒ�л�����ȷ���������� ��Ϊ��ȷ�����Ƿ���ڣ��ɽ�ʵ����иĽ�����Ľ����� ��
��1���� BaSO4 ��2��Na2SO4��Na2CO3
��3��H+��Na+ ��4�� BaCO3+2HNO3=Ba(NO3)2+H2O+CO2��
��5�� NaCl ��������е� BaCl2��Һ��Ϊ Ba(NO3)2��Һ�������������Ȼ�г������ɣ���NaCl���ڣ�������
���������������1�����ݷ�Ӧ�Ĺ��̣��ڷ�Һ��Ʒ�м�����BaCl2��Һ�����ְ�ɫ����A��������BaSO4��BaCO3�������ż�����ϡ���ᣬ��ַ�Ӧ����������ܽ⣬�����������ɣ������弴������̼��˵�����ֳ���BaSO4��BaCO3�����ڣ����Գ����£����� C ˮ��Һ�� pHС��7������ E ��BaSO4
��2����Ȼ����ҺB�м���������������������ɫ������˵����ҺB�к��������ӣ�������˵����Һ�������ӣ���Ϊ��ʵ����м�����BaCl2��Һ�������������ӣ����Է�Һ��һ�����е������ǣ�Na2SO4��Na2CO3
��3����ΪNa2SO4��Na2CO3������������Na+������ʵ��ۼ��������ᣬ����H+��������Һ F ��һ�����е��������ǣ�H+��Na+
��4��ʵ��ڼ�����ϡ��������������ɣ����Է�����Ӧ�Ļ�ѧ����ʽΪ��BaCO3+2HNO3=Ba(NO3)2+H2O+CO2��
��5�����ڷ�Һ��ȷ������Na2CO3���ʿ���ȷ��������CaCl2����Ϊ�������Ӧ�����Է�Һ��Ʒ�л�����ȷ���������ǣ�NaCl��Ϊ��ȷ�����Ƿ���ڣ��ɽ�ʵ����иĽ��������ǣ���������е� BaCl2��Һ��Ϊ Ba(NO3)2��Һ�������������Ȼ�г������ɣ���NaCl���ڣ�������
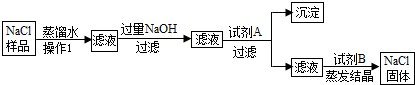
���㣺CO32-��SO42-��Cl-�ļ���

 ��У����ϵ�д�
��У����ϵ�д�ijͬѧ��������ͼ��ʾ��ʵ�飬��14.6%��ϡ�����м���̼��ƣ������10.6%��̼������Һ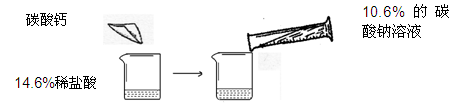
| | ��һ�� | �ڶ��� |
| 14.6%��ϡ��������� | m | m |
| ����̼��Ƶ����� | 10g | 20g |
| ����10.6%��̼������Һ������ | 100g | 200g |
| ����̼������Һ��ʵ������ | ֻ������ | ֻ�а�ɫ���� |
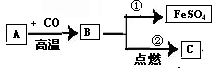
д��ʵ��һ�з�����ѧ��Ӧ�ķ���ʽ
��һ��ʵ���м���̼��ƺ���Һ�����ʳɷ�
������֪�����г����ڶ���ʵ�����ɳ����������ı���ʽ
ʵ���м���ϡ����m������Ϊ
�����ڶ��η�Ӧ�����Һ����191.2gˮ�������ò�������Һ�����ʵ���������Ϊ
����������������29.2%��Ũ��������ʵ�������ϡ���ᣬ����Ҫ��ˮ������
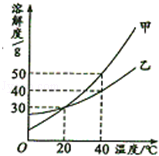
��ͼ�мס��ҡ����dz��л�ѧ�г��������ʣ�ͼ�С�������ʾ����������֮���������Һ�з�����ѧ��Ӧ����������ʾ��ij�����ʿ�ת��Ϊ��һ�����ʣ����ַ�Ӧ������P��Ӧ��������ȥ��������˵���в���ȷ����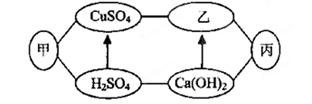
| A��������ֻ������������� |
| B���ס��ҡ�����������ΪFe��NaOH��CO2 |
| C������ΪNaOHʱ��������Ca(OH)2��Na2CO3��Ӧ���� |
| D������Ϊһ�ּ�ʱ����������ͭ��Һ�ķ�Ӧ���ܲ������ֳ��� |