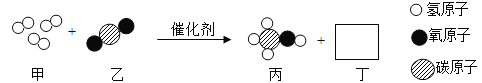
��Ŀ����
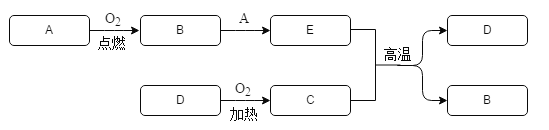
����Ŀ��ijͬѧΪ��̽������Ŀ����ͺ�����������������������̼��ˮ�����ĺ����Ƿ���ͬ������˼�ʵ�鷽��������Ҫ����������ͼ��ʾ�������ͼʾ�ش����⡣
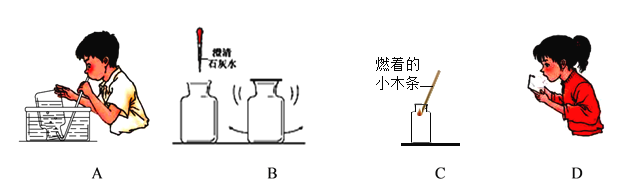
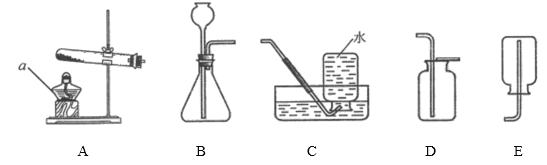
��1����һ��������ͼA����д����ʵ�鲽�貢������ȷ�IJ���˳������Ϊ_________��
�ٰ�ʢ��ˮ��ƿ����ͬ����Ƭһ������ˮ����
����ˮ�½�����ƿ�Ǻã�ȡ��������ʵ��̨��
���ò���Ƭ��______________���ƶ�����Ƭ_________
�ܽ�����ƿʢ��ˮ
�ݽ����Ϲ�С�ĵز��뼯��ƿ�ڻ���������ֱ������ƿ�ڳ�����������
����ˮ�������ò���Ƭ������ƿ��ƿ�ڸǺã�ȡ������ƿ����ʵ������
��2������ͼʾ�г���һ�������������____________
��3��̽����������������ж�����̼�ĺ���ʱ���ֱ�������ͺ�������ļ���ƿ�У������_________����___________�������������ݢ�______�ж϶�����̼������ͬ��
��4��̽����������������������ĺ�����ʵ�飬��۲쵽����������ȼ�ŵ�Сľ����_________������������ȼ�ŵ�Сľ����_______
��5��̽�����������������ˮ�����ĺ���ʵ�飬��õ��Ľ��ۣ�����������ˮ�����ĺ����ȿ�����ˮ�����ĺ���_____��
���𰸡��ܢۢ٢��� ��ס����ƿ�ı�Ե ���꼯��ƿ�� ��ȼ�ŵ�ľ�����ڼ���ƿ�� ����ʯ��ˮ �� ����ʯ��ˮ����ǵij̶� ����ȼ�� Ϩ�� ��
��������
��1����ȷ��˳��Ϊ���ܢۢ٢ݢޣ�
���ò���Ƭ�ȸ�ס����ƿ�ı�Ե���ƶ�����Ƭ���꼯��ƿ�ڣ������ס����ƿ�ı�Ե ���꼯��ƿ�ڡ�
��2��ͼC������������ʱ��Ӧ����ȼ�ŵ�ľ�����ڼ���ƿ�ڣ����Ӧ����ȼ�ŵ�ľ�����ڼ���ƿ�ڡ�
��3��̽����������������ж�����̼�ĺ���ʱ���ֱ�������ͺ�������ļ���ƿ�У��������ʯ��ˮ�������ݳ���ʯ��ˮ����ǵij̶��ж϶�����̼������ͬ��
�������ʯ��ˮ��������ʯ��ˮ����ǵij̶ȡ�
��4��̽����������������������ĺ�����ʵ�飬��۲쵽����������ȼ�ŵ�Сľ������ȼ�գ�����������ȼ�ŵ�Сľ��Ϩ��
�������ȼ�գ�Ϩ��
��5��̽�����������������ˮ�����ĺ���ʵ�飬���ַ��ڿ����еIJ���Ƭû��ˮ����֣����Ź����IJ���Ƭ����ˮ����֣�֤������������ˮ�����ĺ����ȿ�����ˮ�����ĺ����ߡ�
����ߡ�

����Ŀ��Ϊ�ⶨijCuSO4��Һ�����ʵ�����������ȡ150g CuSO4��Һ��ƽ����Ϊ���ݣ�ÿ����Ʒ������ͼ��ʾ����ʵ�飬ʵ�����ݼ��±�������㣺
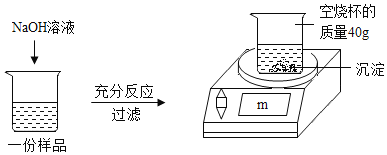
ʵ��1 | ʵ��2 | ʵ��3 | |
��Ʒ����/g | 50 | 50 | 50 |
NaOH��Һ����/g | 20 | 40 | 60 |
m/g | 42.45 | 44.9 | 44.9 |
��1��50g CuSO4��Һ��ȫ��Ӧʱ�����ó���������Ϊ_____g��
��2��CuSO4��Һ�����ʵ���������Ϊ_____��
����Ŀ��ijУ��ѧ��ȤС��ι��Ƽ���������Ϣ���������������о���
����Ʒ��ԭ�����ó������������Ƽ������������Ʒ�������Na2CO3���ͻ���NH4Cl��
����ԭ���ǣ���NH3��CO2ͨ�뱥��ʳ��ˮ�еõ�NaHCO3��NH4Cl�Ļ�����ӦΪ��NaCl�����ͣ�+ NH3 + CO2 + H2O = NaHCO3��+ NH4Cl�������NaHCO3���壬�����Ƶô��
���������̣�

��������ϣ�
��1�� NH4Cl ![]() NH3��+HCl��
NH3��+HCl��
��2�� ��֪20��ʱ�й����ʵ��ܽ�����£�����ָ1���ˮ�����ܽ�����������
���� | NaCl | NaHCO3 | NH4Cl | NH3 | CO2 |
�ܽ�� | 36.0g | 9.6g | 37.2g | 710 | 0.9 |
���������ۣ�:
��1�����ҵ��ؼ���һ���ǣ��ڼ�ѹ�������²����͵İ���ˮ��ͨ�������̼���壬��Һ�л���̼�����ƾ����������Է�����������Ҫ��ѹ������ԭ���ǣ�________�������м��������������Ŀ����________������II�����ƽ�________��
��2����Ӧ���з�����������Ӧ��д�����е�һ����ѧ����ʽ��________________��
��3����Ӧ���еõ�����Ļ�ѧ����ʽΪ��_________________________________��
��4���������������п�ѭ��ʹ�õ���________������ţ���
A ����C B ��ҺD C ������þ D ����NH4Cl
��5���ڰ��ҵ����ʣ����Ȼ����Һʱ��Ϊ�β�ֱ�������ᾧ�����Ȼ�粒���?_________��
�����ȷ����
��1����ȡһ�������Ĵ�����Ʒ������γ�ּ��Ⱥ��ٳ��أ������ޱ仯��
��2����ȡ����������Ʒ��������ˮ����Ʒ��ȫ�ܽ⣬�����Һ�м������ϡHNO3���ٵμ�AgNO3��Һ���а�ɫ������������ʵ���ȷ��������Ʒ��������________��д��ѧʽ����
��3��ij�о���ѧϰС��Ը��������Ĵ����Ʒ���м�⡣ȡ22�˸���Ʒ���ձ��У���ˮ�����ܽ⣬Ȼ����μ���������������Ϊ14.6%��ϡ���Ტ������
���ȷ����ķ�Ӧ�ǣ�Na2CO3+HCl=NaHCO3+NaCl��Ȼ�����ķ�Ӧ�ǣ�NaHCO3+HCl= NaCl+H2O+CO2����
�������������ձ�����Һ���������ϡ���������Ĺ�ϵ��ͼ����ʾ��

�������ͼ���ṩ����Ϣ���������Ӧ�����Ķ�����̼������________���������Ʒ�д������������________(�������ðٷ�����ʾ,������С�����һλ)
����Ŀ��һ�������£������������ܱ������ڳ�ַ�Ӧ����÷�Ӧǰ������ʵ��������£�
������ | �Ҵ� | ���� | ������̼ | ˮ | X |
��Ӧǰ����/g | 2.3 | 4 | 0 | 0 | 0 |
��Ӧ������/g | 0 | 0 | 2.2 | 2.7 | ���� |
A����Ӧ��X������Ϊ1.5g B��X��һ������̼Ԫ�غ���Ԫ��
C��X��һ������̼Ԫ�غ���Ԫ�� D��X������Ԫ�ص���������1��1