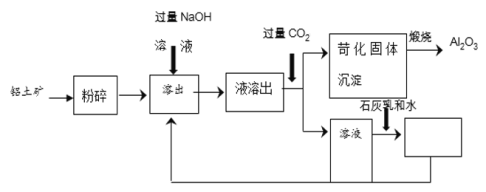
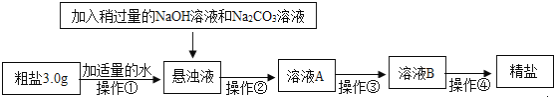
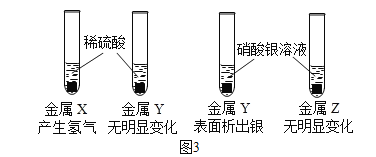
��Ŀ����
����Ŀ����1�������������ڿ�ʯ�У����������ڳ�����Fe2O3����������Fe3O4����������̼����������ѧʽΪ___________����ͭ�����ڻ�ͭ��CuFeS2������ͭ��Cu2S���������Ǹ����ۣ���������Ϊ____________���ȡ�Cu2S��ͭԪ�ص���������Ϊ__________����500�ֺ�Cu2S 80����ͭ��ʯ�������Ͽ�������___________��ͭ��
��2�����������ڹ�ҵ��������Ӧ�Ļ�ѧ����ʽ��______________________________��Fe3O4������Ͽɿ���Fe2O3��FeO������������������ᷴӦ����һ��ʹ��Һ�ʻ�ɫ�����ʺ�һ��ʹ��Һ��dz��ɫ�����ʣ�����һ����ѧ����ʽ��ʾ�÷�Ӧ_________________��
���𰸡�FeCO3 ����ͭ 80% 320 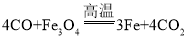 Fe3O4+8HCl=2FeCl3+ FeCl2+4H2O
Fe3O4+8HCl=2FeCl3+ FeCl2+4H2O
��������
��1��̼��������̼�����-2�ۣ���Ϊ��������������Ԫ����+2�ۣ���˻�ѧʽΪ��FeCO3��������֪��ͭ���к�Cu2S���������Ǹ����ۣ���ͭ��+1�ۣ���ͭ��+2�ۺ�+1�����ּ�̬�����Cu2S����Ϊ������ͭ��Cu2S��ͭԪ�ص���������Ϊ��![]() �����ݻ�ѧ��Ӧǰ��Ԫ�ص��������䣬Ԫ�ص�����=���ʵ���������Ԫ�ص�������������500�ֺ�Cu2S 80����ͭ��ʯ�������Ͽ�������ͭ������Ϊ��500����80%��80%=320�֡�
�����ݻ�ѧ��Ӧǰ��Ԫ�ص��������䣬Ԫ�ص�����=���ʵ���������Ԫ�ص�������������500�ֺ�Cu2S 80����ͭ��ʯ�������Ͽ�������ͭ������Ϊ��500����80%��80%=320�֡�
��2����Ϊ���������Ҫ�ɷ���Fe3O4����ҵ��������ԭ�����ڸ��������£��û�ԭ��CO�����������������л�ԭ���������Է�Ӧ�Ļ�ѧ����ʽ�ǣ�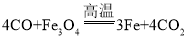 ����ΪFe3O4������Ͽɿ���Fe2O3��FeO���������Fe2O3�������ᷴӦ����һ��ʹ��Һ�ʻ�ɫ������FeCl3��FeO�������ᷴӦ����һ��ʹ��Һ��dz��ɫ������FeCl2�����Խ���������Ӧ�ϲ�����д��һ����ʽ�������һ����ѧ����ʽ��ʾ�ĸ÷�Ӧ�ǣ�Fe3O4+8HCl=2FeCl3+ FeCl2+4H2O��
����ΪFe3O4������Ͽɿ���Fe2O3��FeO���������Fe2O3�������ᷴӦ����һ��ʹ��Һ�ʻ�ɫ������FeCl3��FeO�������ᷴӦ����һ��ʹ��Һ��dz��ɫ������FeCl2�����Խ���������Ӧ�ϲ�����д��һ����ʽ�������һ����ѧ����ʽ��ʾ�ĸ÷�Ӧ�ǣ�Fe3O4+8HCl=2FeCl3+ FeCl2+4H2O��

 �Ƹ������������ϵ�д�
�Ƹ������������ϵ�д�