��Ŀ����
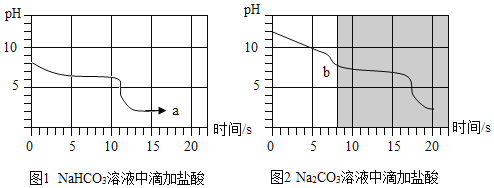
����Ŀ�����ſ�ѧ�ķ�չ������Դ�Ŀ�������ȡ��ͻ�ơ��廪��ѧ�о���Ա�ɹ����Ƴ�һ��������ά�������ɽ�������̼ת����Һ��ȼ�ϼ״�������ʾ��ͼ����ͼ��ͼ�е���ǡ ����ȫ��Ӧ����ʾ���������ʾ��ͼ�ش��������⡣
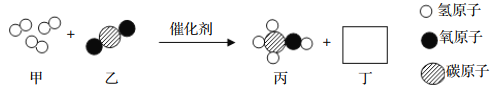
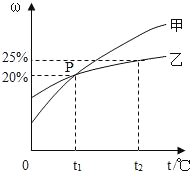
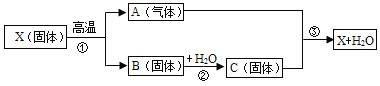
��1�����Ļ�ѧʽΪ______���÷�Ӧ�Ļ�ѧ����ʽΪ______��
��2������˵����ȷ����______����д��ĸ��ţ���
A����Ӧǰ��ԭ����Ŀ�����ı�
B���÷�Ӧ���ڸ��ֽⷴӦ
C�����ǵ��ʣ��ҡ���������Ϊ������
D���÷�Ӧ������������һ�������¿���ת��Ϊ�л���
���𰸡� H2O�� 3H2+CO2![]() CH4O+H2O (��3H2+CO2
CH4O+H2O (��3H2+CO2![]() CH3OH+H2O)�� CD
CH3OH+H2O)�� CD
����������1�����ݷ�Ӧ����ʾ��ͼ�������غ㶨�ɷ����������2�����ݷ�Ӧ�Ļ�ѧ����ʽ�����������Ŀ��Ϣ�������غ㶨�ɿ�֪���÷�Ӧ�ķ���ʽΪ��������̼�������ڴ��������·�Ӧ���ɼ״���ˮ�Ļ�ѧ����ʽΪ3H2+CO2![]() CH4O+H2O (��3H2+CO2
CH4O+H2O (��3H2+CO2![]() CH3OH+H2O)����1����������Ӧ��֪�����Ļ�ѧʽΪH2O���÷�Ӧ�Ļ�ѧ����ʽΪ3H2+CO2
CH3OH+H2O)����1����������Ӧ��֪�����Ļ�ѧʽΪH2O���÷�Ӧ�Ļ�ѧ����ʽΪ3H2+CO2![]() CH4O+H2O (��3H2+CO2
CH4O+H2O (��3H2+CO2![]() CH3OH+H2O)����2��A����������Ӧ��֪����ѧ��Ӧǰ��ԭ����Ŀû�з����ı䣬����B����������Ӧ��֪���÷�Ӧ�����ڸ��ֽⷴӦ������C�������Ĺ��ɿ�֪�����ǵ��ʣ��ҡ���������Ϊ�������ȷ��D����������Ӧ���ʵı仯��֪���÷�Ӧ�������������ת��Ϊ�л����ȷ����ѡCD��
CH3OH+H2O)����2��A����������Ӧ��֪����ѧ��Ӧǰ��ԭ����Ŀû�з����ı䣬����B����������Ӧ��֪���÷�Ӧ�����ڸ��ֽⷴӦ������C�������Ĺ��ɿ�֪�����ǵ��ʣ��ҡ���������Ϊ�������ȷ��D����������Ӧ���ʵı仯��֪���÷�Ӧ�������������ת��Ϊ�л����ȷ����ѡCD��

 �����Ƹ���ʦ����ϵ�д�
�����Ƹ���ʦ����ϵ�д� ��ͨ����ͬ����ϰ��ϵ�д�
��ͨ����ͬ����ϰ��ϵ�д�