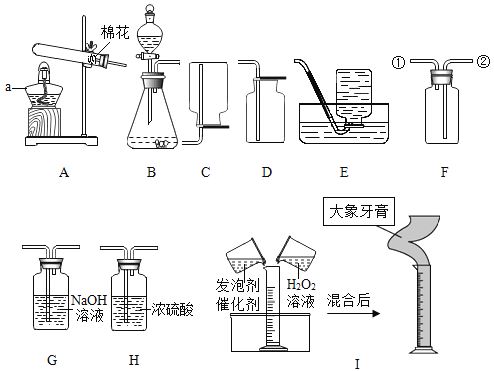
��Ŀ����
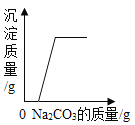
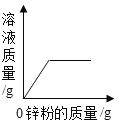
����Ŀ����֪�ձ���װ��200g���������ͭ�����Һ�����к�����ͭ4.8g�����ձ�����������ͭ�����裬�ձ�����Һ���������������ͭ��������ϵ����ͼ��ʾ����ǡ����ȫ��Ӧʱ�����õ���ҺΪ��������Һ��
��1��m=_____g��
��2����ԭ�����Һ��H2SO4����������_____��д��������̣���
��3��ǡ����ȫ��Ӧʱ������Һ�к�������ͭ������Ϊ_____g��
��4��ȡͼ����a���Ӧ����Һ������8%������������Һ���뻭�������������������������������Һ�������Ĺ�ϵ����_____��
���𰸡�208 4.9% 20.8 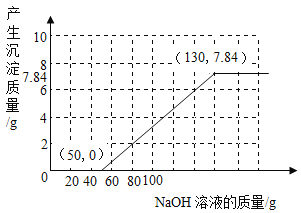
��������
��1������ͭ�������ᷴӦ��������ͭ��Һ����m=200g+8g=208g��
��2���⣺������Һ��H2SO4������Ϊx����������ͭ������Ϊy��
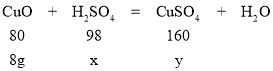
![]() =
=![]() x=9.8 g
x=9.8 g
![]() =
=![]() y=16g
y=16g
ԭ�����Һ��H2SO4����������Ϊ ![]() ��100%=4.9%
��100%=4.9%
��3��ǡ����ȫ��Ӧʱ������Һ�к�������ͭ������Ϊ4.8g+16g=20.8g
��4��ȡͼ����a���Ӧ����Һ������4g����ͭʱ���������������Ϊ4.9g����������ͭΪ8g����ʱ��Һ������ͭ������Ϊ8g+4.8g=12.8g��ʣ��4.9g����δ�μӷ�Ӧ��
�裺4.9g����ǡ��������������Һ�к�ʱ�������������Ƶ�����Ϊz��
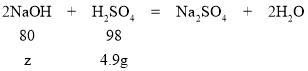
![]() z=4g��
z=4g��
�����кͷ�Ӧʱ����Ҫ��������������Һ������Ϊ=![]() ��
��
������������������Һ��
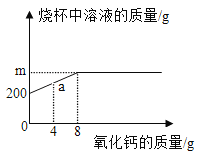
�裺����������ͭ����������Ϊm�������������Ƶ�����Ϊn��
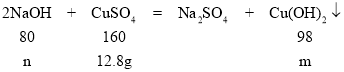
![]() n=6.4g
n=6.4g
![]() m=7.84g
m=7.84g
��������������Һ������=![]() ���ۼ���������������Һ������=50g+80g=130g��
���ۼ���������������Һ������=50g+80g=130g��
�����������������������������Һ�������Ĺ�ϵ����Ϊ��


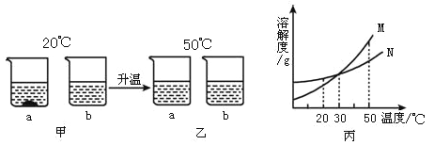
����Ŀ���Ӻ�ˮ�õ��Ĵ����������п��������ʣ��Ȼ�þ���Ȼ��Ƶȣ��Ͳ��������ʣ���ɳ�ȣ���������з�����ᴿ��������ڹ�ҵ���������ǵ��ճ����ʵ����ģ�ҵ�����ᴿ��������ͼ����ش�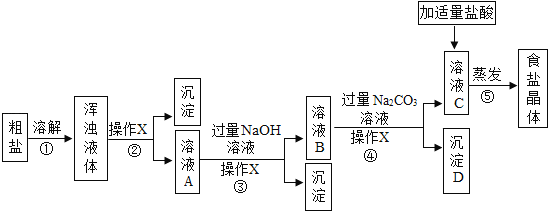
��1������ٺ͢ڵ�Ŀ����________������۵���ҪĿ����________��
��2������X��������________���ò������õ��IJ����������ձ�����������________������ҺC�м����������ᣬ�������������IJ���������________��
��3����ʵ�����ҺC�õ����Σ����õ��������ܼ��ķ��������ý�����Һ�¶ȵķ������ο��ܽ�����ݷ���ԭ��________��
��4���±��ṩ���������ʵ��ܽ�����ݣ���ȡ��Ϣ��ش�
�¶ȡ� | 10 | 20 | 30 | 40 | 50 | 60 | |
�ܽ��/g | NaCl | 35.8 | 36.0 | 36.3 | 36.6 | 37.0 | 37.3 |
KNO3 | 20.9 | 31.6 | 45.8 | 63.9 | 85.5 | 110 |
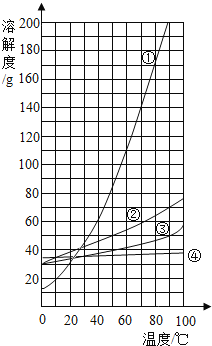
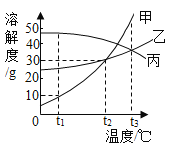
��ͼ�жϣ��Ȼ��Ƶ��ܽ����������ͼ________�������ֱ�ţ���40��ʱ����60g���������100gˮ�У�������10��ʱ����������ؾ���________ g��