��Ŀ����
ij����С��Ϊ�ⶨ����ʯ��ʯ�к�̼��Ƶ�����������ȡ����һЩ��ʯ��Ʒ����ȡϡ����200g��ƽ���ֳ�4�ݣ����ķݲ�ͬ��������Ʒ���뵽�ķ�ϡ�����У�����ʵ�飬������£�
��1���ϱ���m����ֵ��__________________��
��2���Լ�������ʯ��ʯ����̼��Ƶ�����������
��3�������η�Ӧ��������Һ���������������Ƕ��٣�
| ʵ�� | 1 | 2 | 3 | 4 |
| ������Ʒ������/g | 5 | 10 | 15 | 20 |
| ����CO2������/g | 1��54 | 3��08 | 4��4 | m |
��1���ϱ���m����ֵ��__________________��
��2���Լ�������ʯ��ʯ����̼��Ƶ�����������
��3�������η�Ӧ��������Һ���������������Ƕ��٣�
��1��4��4�� ��2��70% ��3��20%
����������Աȱ������ݿ�֪��ʵ��1��ʵ��2�е�����û����ȫ��Ӧ�������е�̼��Ʒ�Ӧ��ȫ��ʵ��3��ʵ��4�е����ᷴӦ��ȫ�����е�̼���û�з�Ӧ��ȫ�����Ե�4��ʵ���е�m��Ȼ��4��4g����5gʯ��ʯ��������̼��Ƶ�����Ϊx����
CaCO3+2HCl=CaCl2+H2O+CO2��
100 44
x 1��54g
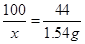
x=3��5g
ʯ��ʯ����̼��Ƶ���������=3��5g/5g��100%=70%��������η�Ӧ�����Ȼ��Ƶ�����Ϊy���μӷ�Ӧ��̼��Ƶ�����Ϊz����
CaCO3+2HCl=CaCl2+H2O+CO2��
100 111 44
z y 4��4g
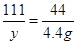
y=11��1g
100/z = 44/4��4g z=10g
�����η�Ӧ��������Һ��������������=
 ��100%=20%��
��100%=20%��
��ϰ��ϵ�д�
�����Ŀ

 2CuSO4+2H2O������һ�������ʵ���������Ϊ9.8%��ϡ����ǡ�ô���2000g��ͭ3.2%�ķ��ϣ������������ʲ������ᷴӦ�Ҳ�����ˮ������Ӧ����������ͭ��Һ�����ʵ�����������
2CuSO4+2H2O������һ�������ʵ���������Ϊ9.8%��ϡ����ǡ�ô���2000g��ͭ3.2%�ķ��ϣ������������ʲ������ᷴӦ�Ҳ�����ˮ������Ӧ����������ͭ��Һ�����ʵ�����������