��Ŀ����
����Ŀ��ijЩʳƷ�İ�װ���ڣ�����װ�а�ɫ����״�����Сֽ��������д�š����������Ҫ�ɷ�Ϊ��ʯ�ҡ���ijͬѧ��һ�����ÿ����еĸ����������̽����
����ȡ���־��ø������������ˮ�У���ֽ��衢����,�õ���ɫ�������Һ��Ȼ�����Һ������ԺͰ�ɫ������������̽����
��ʵ��̽��һ��̽����Һ�������
��ͬѧ�ⶨ����Һ������ԣ�����Ϊ����Һ��____________�ԡ�
��ʵ��̽������̽�����˺��ɫ��������
��ͬѧ��Ϊ��ɫ�����п��ܺ��е�������CaO��Ca(OH)2��CaCO3��
�������й����ϵ�֪��CaO��һ�ְ�ɫ���壬�׳���ʯ�ң�����ˮ��Ӧ�����������ƣ����ų�������������������ˮ��
�������жϣ�����Ϊ�ù��˺�õ��İ�ɫ������һ�������е�������_______��
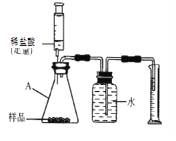
��ʵ������������Ǹ�ͬѧ��ƵĽ�һ��̽����ɫ������ɵ�ʵ�鷽�����������һ�����ʵ�鱨�档
ʵ����� | ʵ������ | ʵ����� |
��ȡ������ɫ���壬��������ˮ����ֽ��衢���ã� �����ϲ���Һ�еμ���ɫ��̪��Һ | �ϲ���Һ��____ɫ | ��ɫ������һ�������������� |
��ȡ������ɫ��������Թ��У��μ�ϡ���� | _________ | ��ɫ������һ������̼��� |
���ܽᷴ˼��
ֻͨ������ʵ�飬������֤����������ڿ����еĸ�������Ƿ���CaO���������һ��֤�����������к��н϶���CaO�ļ���ʵ�飺____���û�ѧ����ʽ��ʾ���еķ�Ӧ_____��
��ʵ��̽������̽���������Ʒ��̼��Ƶ���������
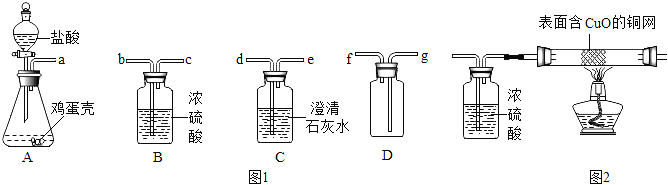
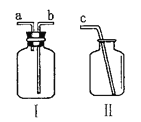
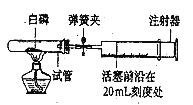
ȡ�����������Ʒ10g������ͼ��װ���У���ַ�Ӧ�������Ͳ�е�ˮ�����Ϊ220mL,(��״���¶�����̼���ܶ���2g/L),��˸������̼��Ƶ���������Ϊ___________(ͨ��������գ�
���ܽᷴ˼����ʵ��̽��������ʵ����ƵIJ�����֮��_________
���𰸡� ���ԡ� �����ƻ�CaO �� ��������� ֤�����������к��н϶���CaO�ļ���ʵ�飺ȡ������Ʒ���Թ��У���ˮ�ܽ⣬�����Թ���ڣ��¶����� CaO+H2O==Ca(OH)2 10% ���ɵĶ�����̼����ˮ����ˮ������Ӧ��ʹ������̼���������С
����������ʵ��̽��һ��������õĸ�����к��������ƣ������������ƣ����������ƺ��������ƣ���������ˮ�У���ֽ��衢���˺����Һ�к����������ƣ���Һ�Լ��ԣ�������õĸ������ֻ����̼��ƣ���������ˮ�У���ֽ��衢���˺����Һ��ˮ�������ԣ��ɷ�����֪������Һ�Լ��Ի���������
�������жϡ��ð�ɫ������һ�������е������������ƣ���Ϊ�������ܺ�ˮ��Ӧ��������������
��ʵ������������ɫ�����к����������ƣ���ô�ϲ���Һ�Լ��ԣ����ֺ�ɫ�������ɫ�����к���̼��ƣ��μ�ϡ����ʱ�����������
���ܽᷴ˼������������ˮ��Ӧ�����������ƣ�Ҳʹ��Һ�Լ��ԣ������ƺ�ˮ��Ӧ�Ļ�ѧ����ʽΪ�� CaO+H2O�TCa��OH��2��֤�����������к��н϶��������Ƶķ����ǣ�ȡ������������Թ��У���������ˮ���������Թܱڣ����ȣ�֤���н϶�������������
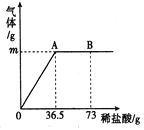
��ʵ��̽�������⣺��������̼��Ƶ�����Ϊx
CaCO3+2HCl =CaCl2 +H2O+CO2��
100 44
x 0.220L��2g/L
![]()
x=1g
�������̼��Ƶ���������Ϊ![]() ��100%=10%
��100%=10%
�𣺸������̼��Ƶ���������Ϊ10%��
�������ɵĶ�����̼����ˮ����ˮ������Ӧ��ʹ������̼���������С������ʵ������д��ڲ������ĵط���

 �ǻ�С��ϰϵ�д�
�ǻ�С��ϰϵ�д�