��Ŀ����
����Ŀ��ͭ��ͭ�Ļ����������������������Ź㷺��Ӧ�á�
��һ��ͭ�Ĺ㷺Ӧ��
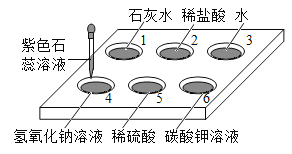
��1���ҹ�������ʱ�ھ��������������Ϊͭ��֮˵���û�ѧ����ʽ��ʾ��ԭ��______��
��2��ͭ�ڹ�ҵ�Ͽ������������£���������˵���Ƥ��������������ͭ��_____�ԡ�
����������ͭ������Ʊ�
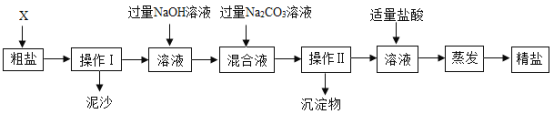
��ҵ���ú�ͭ���ϣ��磺��ͭ������Ƥ�ȣ���ij��ѧС���ͬѧ��������ú�ͭ�����Ʊ�����ͭ���壨CuSO4��xH2O������Ҫ�������£�
![]()
��1����ͭ���Ϸ����Ŀ����__________��
��2���������У�����H2O2��Ҫ���¶ȿ�����50�浽60����ȵ�ԭ��Ϊ_____��
��3����ȤС��ͬѧһ��ָ��������Һ������Ũ����_____����ᾧ�����������˵Ȳ�����������95%�ľƾ���ϴ�����ɣ�������ͭ���壨CuSO4��xH2O����������þƾ���ϴ������ˮ��ԭ����____________��
������ͭ��������ɼ�ʽ̼��ͭ������ҵ�����Ƶõļ�ʽ̼��ͭ����϶࣬����ɱ�ʾΪ��xCuCO3��yCu(OH)2��zH2O��
���ϣ�xCuCO3��yCu(OH)2��zH2O������200ʱ��ֽ�õ�����ͭ��������̼��ˮ��
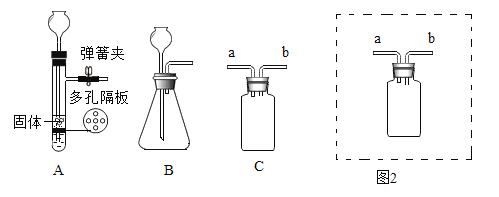
�ⶨij�ֲ�Ʒ����ɣ�����ȤС��ȷ������25.8g����Ʒ�����������װ�ò�����ʵ�飺
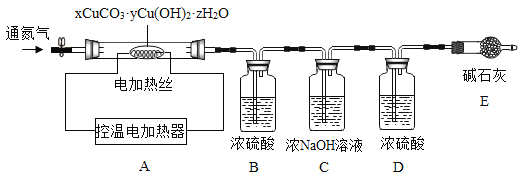
ʵ�����ݼ�¼���±���
B����Һ����/g | C����Һ����/g | D����Һ����/g | E�й�������/g | |
��Ӧǰ | 100.0 | 100.0 | 100.0 | 120.5 |
��Ӧ�� | 105.4 | 104.4 | 100.2 | 120.5 |
ʵ����������ݴ���
��1��ʵ��ǰӦ����_________���ٹ���һ��ʱ�䵪�������B��C��D��E��������
��2��ֹͣ���뵪���������µ����220��������ȣ��۲쵽װ��B��______ʱ������ʽ̼��ͭ�Ѿ���ȫ�ֽ⡣
��3����Ӧ�������ٻ�������һ��ʱ�䵪����Ŀ����___________��
��4���ü�ʽ̼��ͭ�Ļ�ѧʽ��__________��
���𰸡�Fe+CuSO4=FeSO4+Cu ���� ����Ӧ��֮��ĽӴ�������ӿ췴Ӧ���� ��ֹ�¶ȹ��߹�������ֽ� ��ȴ�ᾧ ��ֹ��������ˮ������Ļ�ƾ��лӷ��Ա������ɣ���һ��Ͷԣ� ��������� �������������� ��װ�������壬ʹ���ɵĶ�����̼��ˮ�����������װ�ó������ CuCO3��Cu(OH)2��2H2O
��������
��һ����1�������������Ϊͭ������������ͭ��Ӧ����ͭ���������������Fe+CuSO4=FeSO4+Cu��
��2��ͭ���е����ԣ������Ƴɵ��ߣ���������ԣ�
��������1����ͭ���Ϸ����Ŀ��������Ӧ��֮��ĽӴ�������ӿ췴Ӧ���ʣ��������Ӧ��֮��ĽӴ�������ӿ췴Ӧ���ʣ�
��2��H2O2�����ֽ⣬�¶�Ӧ�ÿ�����50�浽60��֮�䣬��ֹ�¶ȹ��߹�������ֽ⣻�����ֹ�¶ȹ��߹�������ֽ⣻
��3����ȤС��ͬѧһ��ָ��������Һ������Ũ������ȴ�ᾧ�����˵Ȳ����õ�����ͭ���壻��ˮϴ�ӿ����ܽ�����ͭ���壬ʹ����ͭ��������ʧ��������������ͭ������Ҫϴ�Ӻ����ɣ�����ʵ�ϴ���Լ���95%�ľƾ�����Ϊ����ͭ���岻���ھƾ����Ҿƾ��лӷ��Ա������ɣ������ȴ�ᾧ����ֹ��������ˮ������Ļ�ƾ��лӷ��Ա������ɣ�
��������1��ʵ��ǰӦ���ȼ�������ԣ��ٹ���һ��ʱ�䵪�������B��C��D��E�������������������ԣ�
��2��xCuCO3��yCu(OH)2��zH2O������200ʱ��ֽ�õ�����ͭ��������̼��ˮ��ֹͣ���뵪���������µ����220��������ȣ��۲쵽װ��B�в�������������ʱ������ʽ̼��ͭ�Ѿ���ȫ�ֽ⡣����������������ɣ�
��3����Ӧ�������ٻ�������һ��ʱ�䵪����Ŀ������װ�������壬ʹ���ɵĶ�����̼��ˮ�����������װ�ó�����ա������װ�������壬ʹ���ɵĶ�����̼��ˮ�����������װ�ó�����գ�
��4��B����Һ��������5.4g��C����Һ��������4.4g����������ˮ������Ϊ5.4g��������̼������Ϊ4.2g��A����������ͭ���������Ϊ25.8g-4.4g-5.4g=16g��
��̼��ͭ������Ϊx����������ͭ������Ϊy
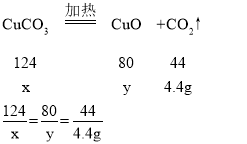
x=12.4g y=8g
����������ͭ���ȷֽ���������ͭ������Ϊ16g-8g=8g
��������ͭ������Ϊz������ˮ������Ϊa
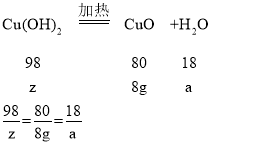
z=9.8g a=1.8g
����xCuCO3��yCu(OH)2��zH2O�нᾧˮ������Ϊ5.4g-1.8g=3.6g
����������![]() x��y��z
x��y��z
ͨ������ɵã�x��y��z=1��1��2
���Ըü�ʽ̼��ͭ�Ļ�ѧʽ��CuCO3��Cu(OH)2��2H2O��
���CuCO3��Cu(OH)2��2H2O��

����Ŀ����� A ��B ��������ѡһ����������������𣬰� A �Ʒ֡�
��һ�������£�Mg �� MgH2���ת������ʵ�������Ĵ�����ͷš��乤��ԭ����ͼ��
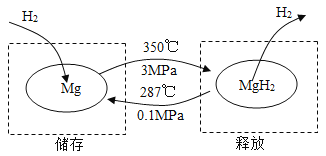
A | B |
��1����������ʱ������Ӧ�Ļ�ѧ����ʽΪ_______�� ��2���������������ݣ��������洢Ч��Ϊ 10%������ 1 kg ������������Ҫ Mg ������Ϊ_______ kg�� | ��1�������ͷ�ʱ������Ӧ�Ļ�ѧ����ʽ Ϊ_______�� ��2�������ϣ�52 kg MgH2������ͷ�����������Ϊ_______ kg�� |
����Ŀ�����±���Ӧ���¶��£���4ֻʢ��100gˮ���ձ��У��ֱ����40 g KCl���壬����ܽ⡣KCl���ܽ����������ͼ
�ձ���� | �� | �� | �� | �� | �� |
�¶ȣ��棩 | 20 | 30 | 40 | 50 | 60 |
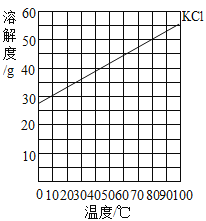
���й��ڢ١���������Һ��˵������ȷ���ǣ���
A.���������٣���
B.����Һͨ�����»�䱥����Һ
C.��������������=��=��
D.�����������ܼ���������Ϊ 2:5