��Ŀ����
����Ŀ�������������У���ᷢ������ѧ������������
(1)ϴ�ྫ��������ۣ�������������_____���á�
(2)�Ϻ������ڰ�װ����ֱ��ˮ����������������̿+���˲�+����������ˮ���ա�����̿�ڴ���_____���ã��������ˮ����_____��ѡ���������������������������
(3) ��ũҵ�����У�ʩ�õIJ�ľ�ң���Ч�ɷ�ΪK2CO3������_____�ʡ�
(4)�ں�������þ������ͭ�Ĺ�ҵ��ˮ�м��������п�ۣ���ַ�Ӧ����ˣ����ù����ǣ���д��ѧʽ��_____��
(5)��ѧΪ��������ѩ����̿�����ҽ������������ء���ٵ���Ŀ���˶�Ա�ڱ���ǰ���ð�ɫ����þ�������֣�������Ϊ��þ�������ᡢ��ˮ�Ժã�������������
��þ��������Ч�ɷ��Ǽ�ʽ̼��þ��������ȼ��300�����ֽ⣬��ֽ�Ļ�ѧ����ʽ�ǣ�
Mg5(OH)2(CO3)4��5MgO+X+4CO2��,��X�Ļ�ѧʽ��_____��������Щ��Ϣ�������ƶϳ���þ��������һ����;��_____��
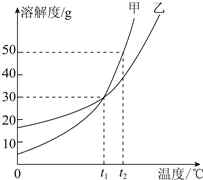
���𰸡��黯 ���� ����� �� Zn��Cu H2O ��ȼ��(��������)
��������
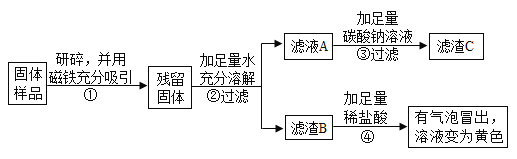
��1��ϴ�ྫ��������ۣ��������������黯���ã�����黯��
��2���Ϻ������ڰ�װ����ֱ��ˮ����������������̿+���˲�+����������ˮ���գ�����̿�ڴ����������ã��������ˮ����Ȼ����һЩ����ˮ�����ʣ����ڻ����������������
��3����ũҵ�����У�ʩ�õIJ�ľ�ң���Ч�ɷ�ΪK2CO3�����ڼطʣ�����أ�
��4��п����ͭ��ǰ�棬þ�ĺ��棬����п�ܺ�����ͭ��Һ��Ӧ��������п��ͭ�����ܺ�����þ��Һ��Ӧ������Ϊп�ǹ����ģ����Գ�ַ�Ӧ����ˣ����ù�����п��ͭ�����Zn��Cu��
��5������Mg5��OH��2��CO3��4![]() 5MgO+X+4CO2����֪��ÿ��X�к���2����ԭ�Ӻ�1����ԭ�ӣ���ˮ�����H2O��
5MgO+X+4CO2����֪��ÿ��X�к���2����ԭ�Ӻ�1����ԭ�ӣ���ˮ�����H2O��
��Ϊ��ʽ̼��þ����ȼ��300�����ֽ������ɶ�����̼�����Կ���������ȼ���������ȼ����

 ��У����ϵ�д�
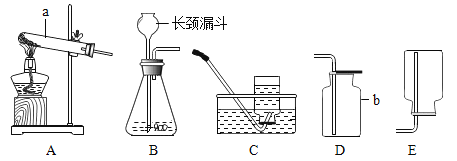
��У����ϵ�д�����Ŀ�������װ�г�ʹ��һ�ִ�װ��������Ʒ��Ϊ��504˫�����������ǩ��ͼ��ʾ��ͬѧ�Ƕ�һ�����õ���504˫������������Ʒ�ܺ��棬���ʵ�����̽����

��������⣩���ù���ijɷ���ʲô��
���������ϣ������Ȼ�����Һ�ڳ����·�����Ӧ�����Ȼ�������
���������룩���ù����п��ܺ���Fe��Fe2O3��CaO��Ca��OH��2��CaCO3��
��ʵ��̽��1����ͬѧ�ķ�����
ʵ����� | ʵ������ | ʵ����� |
��1��ȡ�����������Թ��У��ܽ⣬���ú�ȡ�ϲ���Һ�μ���ɫ��̪��Һ | �����ܽ�ʱ�Թ���ڷ��̣��Թܵײ��в������Һ��� | ������һ����_____���������� |
��2����ȡ�����������Թ��У��μ�������_____ | ��������ʧ���д�����ɫ����������õ�dz��ɫ��Һ | ������һ������______,һ������Fe2O3 |
��3�������裨2���в���������ͨ�뵽�����ʯ��ˮ�� | _______ | ������һ������CaCO3 |
��ʵ�����ɣ�
��1����ͬѧ��Ϊ��ͬѧ��ʵ���в��ܵó�һ����Ca��OH��2�Ľ��ۣ�������_________��
��2����ͬѧ��Ϊ��ͬѧ��ʵ�鲢���ܵó�һ������FeO3�Ľ��ۣ�������_________��
��ʵ��̽��2���ҡ���ͬѧ�������ʵ�鷽��������֤��
��1���ҡ���ͬѧʵ�������ܵó�������Ʒ��һ��������_______������һ�����ʲ���ȷ������������_______��
��2���ҡ���ͬѧ�־���ʵ��������3.9g���������к������ʵ�������Ϊ2.3g������B��CaCO3������Ϊ1.0g������C������Ϊ2.0g��
��ʵ����ۣ��ۺ�����ʵ�鼰�������ݣ�
��1���жϾ��ù���ijɷ���_______��
��2�������������������������Ƶ�����������Ϊ______�ˡ�
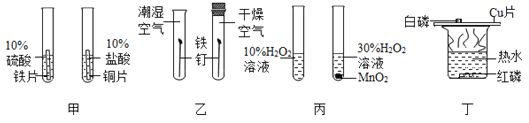
����Ŀ��ͭ��ͭ�Ļ����������������������Ź㷺��Ӧ�á�
��һ��ͭ�Ĺ㷺Ӧ��
��1���ҹ�������ʱ�ھ��������������Ϊͭ��֮˵���û�ѧ����ʽ��ʾ��ԭ��______��
��2��ͭ�ڹ�ҵ�Ͽ������������£���������˵���Ƥ��������������ͭ��_____�ԡ�
����������ͭ������Ʊ�
��ҵ���ú�ͭ���ϣ��磺��ͭ������Ƥ�ȣ���ij��ѧС���ͬѧ��������ú�ͭ�����Ʊ�����ͭ���壨CuSO4��xH2O������Ҫ�������£�
![]()
��1����ͭ���Ϸ����Ŀ����__________��
��2���������У�����H2O2��Ҫ���¶ȿ�����50�浽60����ȵ�ԭ��Ϊ_____��
��3����ȤС��ͬѧһ��ָ��������Һ������Ũ����_____����ᾧ�����������˵Ȳ�����������95%�ľƾ���ϴ�����ɣ�������ͭ���壨CuSO4��xH2O����������þƾ���ϴ������ˮ��ԭ����____________��
������ͭ��������ɼ�ʽ̼��ͭ������ҵ�����Ƶõļ�ʽ̼��ͭ����϶࣬����ɱ�ʾΪ��xCuCO3��yCu(OH)2��zH2O��
���ϣ�xCuCO3��yCu(OH)2��zH2O������200ʱ��ֽ�õ�����ͭ��������̼��ˮ��
�ⶨij�ֲ�Ʒ����ɣ�����ȤС��ȷ������25.8g����Ʒ�����������װ�ò�����ʵ�飺
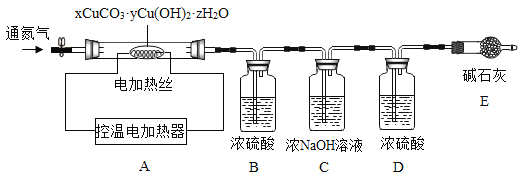
ʵ�����ݼ�¼���±���
B����Һ����/g | C����Һ����/g | D����Һ����/g | E�й�������/g | |
��Ӧǰ | 100.0 | 100.0 | 100.0 | 120.5 |
��Ӧ�� | 105.4 | 104.4 | 100.2 | 120.5 |
ʵ����������ݴ���
��1��ʵ��ǰӦ����_________���ٹ���һ��ʱ�䵪�������B��C��D��E��������
��2��ֹͣ���뵪���������µ����220��������ȣ��۲쵽װ��B��______ʱ������ʽ̼��ͭ�Ѿ���ȫ�ֽ⡣
��3����Ӧ�������ٻ�������һ��ʱ�䵪����Ŀ����___________��
��4���ü�ʽ̼��ͭ�Ļ�ѧʽ��__________��