��Ŀ����
����Ŀ��ij��ѧ��ȤС��Ҫ����100g��������Ϊ20%��̼������Һ����ͼ��������̼������Һ��ʵ�����ʾ��ͼ����ش��������⣺
��1����������100g������������Ϊ20%��̼������Һ���裺̼����_____g��ˮ_____mL����ȡ����Ҫ��ˮӦѡ��_____��ѡ�10 mL������50 mL����100 mL������Ͳ��
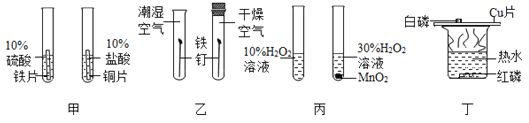
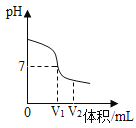
��2��������ͼʾ����ű�ʾ������Һ�IJ���˳��_____��
��3��������̼������Һ�Ĺ�����Ӧ���õ��IJ��������ֱ���_____��
��4�������ȡ��̼�����к�������ˮ�֣�������Ƶ�̼������Һ��Ũ�Ƚ�_____���ƫ�ߡ�����ƫ�͡�������Ӱ�족����
��5����������ƽ��ȷ���������̼����ʱ������������ƽ��ָ��ƫ�����̣�Ӧ_____������ţ�
A ����������̼���ƹ��� B ����������̼���ƹ��� C ����ƽ����ĸ D ��������
���𰸡�20 80 100mL C��B��D��E��A �ձ����������ͽ�ͷ�ι� ƫ�� B
��������
���������и�ʴ�����ʲ���ֱ�ӷ��ڳ���ֽ�ϣ���Ҫ���ڲ��������ϣ�����ȡ��ʱѡ����ȡ��Χ�ӽ�����Ͳ����ȷ��ȷ�ԣ��ܽ�ʱ��������ձ��У��ò���������ʹ���ʳ���ܽ⣻��װƿ��š�ʱ�����ƺõ���Һװ���Լ�ƿ���Ǻ�ƿ�Dz����ϱ�ǩ�������Լ����С�
��1������100g������������Ϊ20%��̼������Һ���裺̼���Ƶ�����Ϊ![]() ��ˮ������Ϊ
��ˮ������Ϊ![]() ����ˮ�����Ϊ80mL����ȡҺ�����ѡ���Һ������ӽ��Ŀ̶ȣ�������Ҫ��ˮӦѡ��100mL��Ͳ��
����ˮ�����Ϊ80mL����ȡҺ�����ѡ���Һ������ӽ��Ŀ̶ȣ�������Ҫ��ˮӦѡ��100mL��Ͳ��
��2��������Һ��˳��Ϊ���㡢��������ȡ���ܽ⡢װƿ��ţ���������Һ�IJ���˳��C��B��D��E��A��
��3��������̼������Һ�Ĺ������ܽ���Ҫ�ձ���������Ҫ���������μ�Һ����Ҫ��ͷ�ιܣ���Ӧ���õ��IJ��������ֱ����ձ����������ͽ�ͷ�ιܡ�
��4�������ȡ��̼�����к�������ˮ�֣���Һ�������������Ƶ�̼������Һ��Ũ�Ƚ�ƫ�͡�
��5����������ƽ��ȷ���������̼����ʱ������������ƽ��ָ��ƫ�����̣�˵��̼���Ƶ�����̫���ˣ���Ӧ�ü���������̼���ƹ��壬��ѡB��

����Ŀ��ͭ��ͭ�Ļ����������������������Ź㷺��Ӧ�á�
��һ��ͭ�Ĺ㷺Ӧ��
��1���ҹ�������ʱ�ھ��������������Ϊͭ��֮˵���û�ѧ����ʽ��ʾ��ԭ��______��
��2��ͭ�ڹ�ҵ�Ͽ������������£���������˵���Ƥ��������������ͭ��_____�ԡ�
����������ͭ������Ʊ�
��ҵ���ú�ͭ���ϣ��磺��ͭ������Ƥ�ȣ���ij��ѧС���ͬѧ��������ú�ͭ�����Ʊ�����ͭ���壨CuSO4��xH2O������Ҫ�������£�
![]()
��1����ͭ���Ϸ����Ŀ����__________��
��2���������У�����H2O2��Ҫ���¶ȿ�����50�浽60����ȵ�ԭ��Ϊ_____��
��3����ȤС��ͬѧһ��ָ��������Һ������Ũ����_____����ᾧ�����������˵Ȳ�����������95%�ľƾ���ϴ�����ɣ�������ͭ���壨CuSO4��xH2O����������þƾ���ϴ������ˮ��ԭ����____________��
������ͭ��������ɼ�ʽ̼��ͭ������ҵ�����Ƶõļ�ʽ̼��ͭ����϶࣬����ɱ�ʾΪ��xCuCO3��yCu(OH)2��zH2O��
���ϣ�xCuCO3��yCu(OH)2��zH2O������200ʱ��ֽ�õ�����ͭ��������̼��ˮ��
�ⶨij�ֲ�Ʒ����ɣ�����ȤС��ȷ������25.8g����Ʒ�����������װ�ò�����ʵ�飺
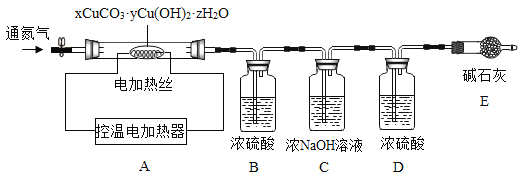
ʵ�����ݼ�¼���±���
B����Һ����/g | C����Һ����/g | D����Һ����/g | E�й�������/g | |
��Ӧǰ | 100.0 | 100.0 | 100.0 | 120.5 |
��Ӧ�� | 105.4 | 104.4 | 100.2 | 120.5 |
ʵ����������ݴ���
��1��ʵ��ǰӦ����_________���ٹ���һ��ʱ�䵪�������B��C��D��E��������
��2��ֹͣ���뵪���������µ����220��������ȣ��۲쵽װ��B��______ʱ������ʽ̼��ͭ�Ѿ���ȫ�ֽ⡣
��3����Ӧ�������ٻ�������һ��ʱ�䵪����Ŀ����___________��
��4���ü�ʽ̼��ͭ�Ļ�ѧʽ��__________��