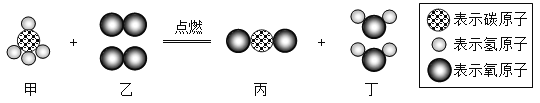
��Ŀ����
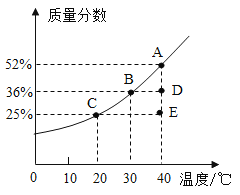
����Ŀ����ͼΪ��������X�ı�����Һ�¶��������������ı仯���ߡ�
��ش���������:
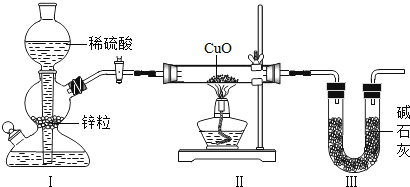
(1)����E�����Һ������30�棬������ҺΪ___________(���� ������ ���� ��������)��Һ��
(2) ����D�����Һ���������ܼ�ʱ��D�㽫��_____���ƶ�(ѡ��A��E) ��
(3)����50gA�����Һ������20�棬����Һ������X������Ϊ_________��
(4) 30��ʱ����Ҫʹmg��������Ϊ30%��X��Һ��Ϊ36%�����õķ���������_____ (�����) ��
a������m/6gˮ
b������ԭ��Һ���ܼ�������1/2
c���ܽ�0.005mg��X��ĩ
d����1.2mg40%��X��Һ
���𰸡������� A 18g a
��������
��1�����ݹ�������X�ı�����Һ�¶��������������ı仯���ߣ�E���ʾ����X���¶�Ϊ40������������Ϊ25%�IJ�������Һ��30��ʱ����X�ı�����Һ����������36%������E�����Һ������30�棬��ʱ�õ����Dz�������Һ��
��2������D�����Һ���������ܼ������������¶Ȳ�����Ϊ40�棬�����ܼ������ʵ����������������D�㽫��A���ƶ���
��3��A���ʾ����X���¶�Ϊ40������������Ϊ52%�ı�����Һ��50gA�к��е����ʵ�����Ϊ50g��52%=26g���ܼ�������Ϊ50g-26g=24g������50gA�����Һ������20�棬�¶Ƚ��ͣ��ܼ����䣬X�����ܽ�ȱ�С��20��ʱ����X�ı�����Һ����������25%����24g�ܼ�������ܽ�����Ϊ![]() ����
����![]() �����
�����![]() =8g������Һ������X������Ϊ26g-8g=18g��
=8g������Һ������X������Ϊ26g-8g=18g��
��4�����ݹ�������X�ı�����Һ�¶��������������ı仯���ߣ�30��ʱ��X���ʱ�����Һ����������Ϊ36%����Ҫʹmg��������Ϊ30%��X��Һ��Ϊ36%�����õķ��������������ܼ����������ʡ�
�������ܼ������������ʵ��������䣬mg��������Ϊ30%��X��Һ�����ʵ�����Ϊ30%��mg=0.3 mg��ˮ������Ϊmg-30%��=0.7 mg����Ϊ36%�ı�����Һ����Һ������Ϊ![]() ��ˮ������Ϊ
��ˮ������Ϊ![]() ������������������Ҫ����ˮ������Ϊ
������������������Ҫ����ˮ������Ϊ![]() ��������ԭ��Һ���ܼ�������
��������ԭ��Һ���ܼ�������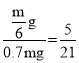 ��
��
���������ʣ��������ܼ����������䣬mg��������Ϊ30%��X��Һ�����ʵ�����Ϊ30%��mg=0.3 mg��ˮ������Ϊmg-30%��=0.7 mg����Ϊ36%�ı�����Һ����Һ������Ϊ![]() �����ʵ�����Ϊ
�����ʵ�����Ϊ![]() ���������ӵ����ʵ�����Ϊ
���������ӵ����ʵ�����Ϊ![]()
������1.2mg40%��X��Һ����������ʵ�����Ϊ0.3 mg+1.2mg��40%=0.78 mg����Һ������Ϊmg+ 1.2mg=2.2 mg�����ʵ���������Ϊ![]()
��ѡa��

 ��������ܸ�ϰϵ�д�
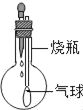
��������ܸ�ϰϵ�д�����Ŀ��̽��С��������ͼװ�ý�������ʵ�飬��ͷ�ι�ʢװ����Һ�壬��ƿ��ʢװ�������塢�����Һ�壬����ͷ�ιܵμ���Һ����ܹ۲쵽�������ʵ������ǣ�������
��� | �ι��� | ��ƿ�� |
A | Ca��OH��2 | O2 |
B | HCl | Zn���̣� |
C | NaOH | CO2������ |
D | H2SO4��Ũ�� | H2O��Һ�� |
A. AB. BC. CD. D