��Ŀ����
����Ŀ��ʵ���ҳ��ü�������غͶ������̻����ķ�����ȡ��������ش��������⣺

��1����Ӧ�Ļ�ѧ����ʽΪ________��
��2�����������������![]() ________
________![]() ________��
________��
��3��������������װ��������Ӧ��������װ�ã���ѡ��ķ���װ��Ϊ________��![]() װ���Թܿ�Ҫ��������б��ԭ����________���ռ�װ��Ϊ________��
װ���Թܿ�Ҫ��������б��ԭ����________���ռ�װ��Ϊ________��
����װ��ѡ���������________������ĸ����
![]() ���ڹ̹��ͼ��ȵķ�Ӧ
���ڹ̹��ͼ��ȵķ�Ӧ ![]() ���ڹ�Һ�Ͳ����ȵķ�Ӧ
���ڹ�Һ�Ͳ����ȵķ�Ӧ
![]() ��ȡ�������ܶȱȿ�����
��ȡ�������ܶȱȿ����� ![]() ��ȡ������������ˮ
��ȡ������������ˮ
��4������ͼ��װ�ý������ſ��������ռ���ȡ��![]() �����ͼ�е�������������������_____��
�����ͼ�е�������������������_____��

��5���м�λͬѧһ��̽��������غͶ������̻��Ϊ���ٱ���ʱ�������������ٶ���죮ʵ��ʱ�������¼ʱ�䣬��ʱ�Ӽ��ȵ��ռ���һƿ����Ϊ��ʵ�������õ�װ���ǽ�![]() ��________����������±�Ϊ��������������ز�ͬ������ʱ����ȡ�������ٶȱȽ�
��________����������±�Ϊ��������������ز�ͬ������ʱ����ȡ�������ٶȱȽ�
ʵ����� | ��������������ص������� | ��ʱ���룩 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
��ʵ�����![]() ��
��![]() ���ݿ��Կ�����ʵ�����________����
���ݿ��Կ�����ʵ�����________����![]() ��
��![]() ����Ӧ�ٶ���죮ͨ��������֪���ڻ�ѧ��Ӧ�д�������������������������������________Խ��Խ�ã�
����Ӧ�ٶ���죮ͨ��������֪���ڻ�ѧ��Ӧ�д�������������������������������________Խ��Խ�ã�
��6�����ڷ�Ӧ��IJ��������ᴿ![]() ����������²�������ѿ�ȱ�����ϣ�_____��_____
����������²�������ѿ�ȱ�����ϣ�_____��_____

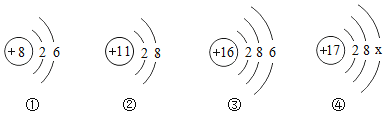
�ڢ١��ڡ��۵IJ����о��õ���������________��
���𰸡�2KClO3![]() 2KCl+3O2�� ��Һ©�� ��ƿ
2KCl+3O2�� ��Һ©�� ��ƿ ![]() ��ֹˮ��������������ը���Թ� C��D ACD
��ֹˮ��������������ը���Թ� C��D ACD  C
C ![]() ���� ���� �����ᾧ ������
���� ���� �����ᾧ ������
��������
��1��������ڶ������̵Ĵ����������ȷֽ�Ļ�ѧ����ʽΪ�� �����
����� ��
��
��2����ͼ��֪a�Ƿ�Һ©���� b����ƿ�������Һ©������ƿ��
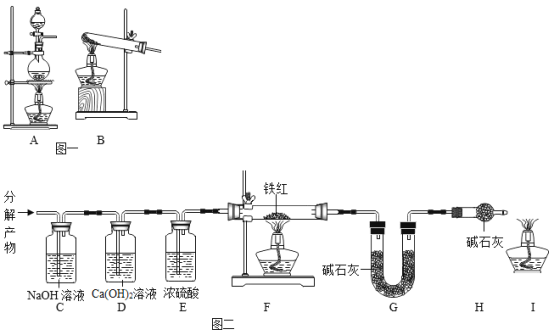
��3�������������ȡ������Ҫ���ȣ�Ӧ����![]() װ����Ϊ����װ�ã�Bװ���Թܿ�Ҫ��������б��ԭ���ǣ��ܹ���ֹ����ˮ������ʹ�Թ����ѣ���Ϊ�������ܶȱȿ��������������ſ������ռ�������Dװ���ռ���������������ˮ����������ˮ���ռ�������Cװ���ռ�������װ��ѡ��������ǣ����ڹ̹��ͼ��ȵķ�Ӧ����ȡ�������ܶȱȿ�������ȡ������������ˮ�����B����ֹˮ��������������ը���Թܣ�C��D��ACD��
װ����Ϊ����װ�ã�Bװ���Թܿ�Ҫ��������б��ԭ���ǣ��ܹ���ֹ����ˮ������ʹ�Թ����ѣ���Ϊ�������ܶȱȿ��������������ſ������ռ�������Dװ���ռ���������������ˮ����������ˮ���ռ�������Cװ���ռ�������װ��ѡ��������ǣ����ڹ̹��ͼ��ȵķ�Ӧ����ȡ�������ܶȱȿ�������ȡ������������ˮ�����B����ֹˮ��������������ը���Թܣ�C��D��ACD��
��4������ͼ��װ�ý������ſ��������ռ���ȡ��![]() �������ܶȱȿ�����ͼ�е�������������ͼ����ʾ��
�������ܶȱȿ�����ͼ�е�������������ͼ����ʾ��

��5��Ϊ�˱��ڹ۲��ռ���һƿ������Ҫ��ʱ�䣬Ӧ������ˮ���ռ�����������Cװ���ռ���������ʵ�����2��3���ݿ��Կ�����ʵ�����2��Ӧ�ٶ���죻ͨ�������������ݿ�֪���ڻ�ѧ��Ӧ�д�������������Խ��Խ�ã����2�����ǡ�
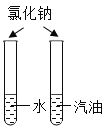
��6���Ȼ���������ˮ���������̲�����ˮ��ͨ�����˿��Ѷ������̳�ȥ���Ȼ�����Һ�����������Եõ��Ȼ��ع��壬�����ͼ����ʾ��

�ڢ��У�ͨ��������������Լӿ��Ȼ��ص��ܽ⣻���У�����ʱӦ���ò��������������У�ͨ���ò��������裬���Է�ֹҺ���⽦������ڢ١��ڡ��۵IJ����о��õ��������Dz������������������

����Ŀ����ѧ�о��г������۲졢�۵��������ֱ�Ӳ�����ͨ��ʵ��ķ���ת��Ϊ�۲졢��۵�������ײ����ģ��˷�����ת����������ʵ���У�û������ת�������ǣ� ��
|
|
|
|
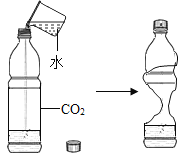
A.̽��ͬ�������ڲ�ͬ�ܼ��е��ܽ��� | B.̽���������������� | C.��a���Ӻ��ԭ���о�ԭ�ӽṹ | D.̽��CO2������ |
A.AB.BC.CD.D