��Ŀ����
����������ͨ���������һ����ɫ���д̼�����ζ���ж����壬����������������Һ��Ӧ�����κ�ˮ������ijһʵ��С���ͬѧ��������ͼװ�ú�ҩƷ��ȡ���������Զ���������ˮ��ͨ��������ܷ�����ѧ��Ӧ����ʵ��̽������Ƶ�̽���������£�����ش����е��й����⣺

�ż��裺����������ˮ��ͨ��������ܷ�����ѧ��Ӧ����������һ���ᡣ
����Ʒ���������֤ˮ�ܷ�ʹ��ɫʯ����ֽ��ɫ������֤�������������ܷ�ʹ�������ɫʯ����ֽ��ɫ�������֤�������������ܷ�ʹʪ�����ɫʯ����ֽ��졣
�Dz������ϣ�����Ϊ���о�С����Ҫ���ĵ�����������Ӧ����________����д��ţ�
a. ��������������ˮ b. ����ʹʪ�����ɫʯ����ֽ���
c. ���������ܱ�Ũ�������
��ʵ�飺
��ʵ������У�װ��C��ʯ����ֽ����ɫʼ��û�б仯����˵��_________________��
��װ��D�н�ͷ�ι��е�����ˮ�ڶ���������������֮ǰ�ε���ɫʯ����ֽ�ϣ�δ����ֽ������ɫ�仯��������˵�� �����ж�����������ͨ��ʱ����ʪ�����ɫʯ����ֽ��죬������˵��____________ ________________��
��װ��E��������___________________________��д��������Ӧ�Ļ�ѧ����ʽ___________________________��
�ɽ��ۣ�ԭ����___________�������������������

�ż��裺����������ˮ��ͨ��������ܷ�����ѧ��Ӧ����������һ���ᡣ
����Ʒ���������֤ˮ�ܷ�ʹ��ɫʯ����ֽ��ɫ������֤�������������ܷ�ʹ�������ɫʯ����ֽ��ɫ�������֤�������������ܷ�ʹʪ�����ɫʯ����ֽ��졣
�Dz������ϣ�����Ϊ���о�С����Ҫ���ĵ�����������Ӧ����________����д��ţ�
a. ��������������ˮ b. ����ʹʪ�����ɫʯ����ֽ���
c. ���������ܱ�Ũ�������
��ʵ�飺
��ʵ������У�װ��C��ʯ����ֽ����ɫʼ��û�б仯����˵��_________________��
��װ��D�н�ͷ�ι��е�����ˮ�ڶ���������������֮ǰ�ε���ɫʯ����ֽ�ϣ�δ����ֽ������ɫ�仯��������˵�� �����ж�����������ͨ��ʱ����ʪ�����ɫʯ����ֽ��죬������˵��____________ ________________��
��װ��E��������___________________________��д��������Ӧ�Ļ�ѧ����ʽ___________________________��
�ɽ��ۣ�ԭ����___________�������������������
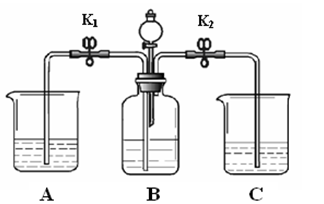
��3��abc ��4���ٶ���������ʹ�������ɫʯ����ֽ��ɫ
��ˮ����ʹ��ɫʯ����ֽ��ɫ��SO2����ˮ��������ʹ��ɫʯ����ֽ���
������SO2��������Ⱦ����
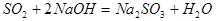 ��5������
��5��������������3���������е�װ��ͼ���о������������Ʒ������з�����
��4���ٸ������ʹ��ɫʯ����ֽ���ɫ���з�����
�ڸ���ˮ����ɫʯ����ֽ�Ϻ�ͨ�������������ֽ����ɫ�仯���з�����
�۸��ݶ��������ж����з�����
��5������������Ƶ����з�����
��𣺽⣺��3������Ƶķ����п�֪Dװ��������ɫʯ����ֽ�͵μ�����ˮ��װ�ã�Bװ����Ũ�����ڸ������壬�ʴ�Ϊ��abc��
��4���ٶ�������������ɫʯ����ֽ�Ӵ�ʼ��û�б仯��˵����������ʹ�������ֽ��ɫ���ʴ�Ϊ��SO2����ʹ�������ɫʯ����ֽ���ɫ��
���ȼ�����ˮû�б�ɫ��˵������ˮ����ʹ��ֽ��ɫ��ͨ�������������ֽ����˺�ɫ����Ϣ��е��Ƶ���֪���Ƕ��������ˮ��������ʹ��ֽ��ɫ�ģ��ʴ�Ϊ��ˮ����ʹ��ɫʯ����ֽ��ɫ��SO2����ˮ��������ʹ��ɫʯ����ֽ��ɫ��
�۶���������һ����ɫ���д̼�����ζ���ж����壬����������������Һ��Ӧ��������������ˮ���ʴ�Ϊ�����ն���SO2���壬������Ⱦ������SO2+2NaOH=Na2SO3+H2O��
��5��ͨ��������Ƶ����Կ���SO2��ˮ��ͨ��������ܷ�����ѧ��Ӧ����������һ���ᣬ�ʴ�Ϊ��������
��4���ٸ������ʹ��ɫʯ����ֽ���ɫ���з�����
�ڸ���ˮ����ɫʯ����ֽ�Ϻ�ͨ�������������ֽ����ɫ�仯���з�����
�۸��ݶ��������ж����з�����
��5������������Ƶ����з�����
��𣺽⣺��3������Ƶķ����п�֪Dװ��������ɫʯ����ֽ�͵μ�����ˮ��װ�ã�Bװ����Ũ�����ڸ������壬�ʴ�Ϊ��abc��
��4���ٶ�������������ɫʯ����ֽ�Ӵ�ʼ��û�б仯��˵����������ʹ�������ֽ��ɫ���ʴ�Ϊ��SO2����ʹ�������ɫʯ����ֽ���ɫ��
���ȼ�����ˮû�б�ɫ��˵������ˮ����ʹ��ֽ��ɫ��ͨ�������������ֽ����˺�ɫ����Ϣ��е��Ƶ���֪���Ƕ��������ˮ��������ʹ��ֽ��ɫ�ģ��ʴ�Ϊ��ˮ����ʹ��ɫʯ����ֽ��ɫ��SO2����ˮ��������ʹ��ɫʯ����ֽ��ɫ��
�۶���������һ����ɫ���д̼�����ζ���ж����壬����������������Һ��Ӧ��������������ˮ���ʴ�Ϊ�����ն���SO2���壬������Ⱦ������SO2+2NaOH=Na2SO3+H2O��
��5��ͨ��������Ƶ����Կ���SO2��ˮ��ͨ��������ܷ�����ѧ��Ӧ����������һ���ᣬ�ʴ�Ϊ��������

��ϰ��ϵ�д�
 ��ѧ����ϵ�д�
��ѧ����ϵ�д�
�����Ŀ


 (4) ����(H2S)��һ����ɫ���г�������ζ���ж����壬��
(4) ����(H2S)��һ����ɫ���г�������ζ���ж����壬��


