��Ŀ����
ij��ѧ��ȤС��Ϊ�˲ⶨһ��ʯ��ʯ��Ʒ��̼��Ƶ�����������ȡ��2gʯ��ʯ��Ʒ����25gϡ�����5�μ�����Ʒ�У��������ʲ������ᷴӦ��Ҳ���ܽ���ˮ������ַ�Ӧ���ˡ�����Ȳ���������������ʵ���������£�
| ϡ��������� | ʣ���������� |
| ��һ�μ���5g | 1.5g |
| �ڶ��μ���5g | 1.0g |
| �������5g | 0.5g |
| ���Ĵμ���5g | X |
| ������5g | 0.3g |
��2����ʯ��ʯ��Ʒ��̼��Ƶ���������Ϊ���٣�
��3���������Ʒ��һ���Լ���15g�����ַ�Ӧ���ˣ���������Һ���ʵ�����������
�⣺��1���ɵ�һ�κ͵ڶ��εķ�Ӧ���Է���5g�����ܹ�����0.5g̼��ƣ������ʣ���0.3g����̼����е����ʣ��������εĹ�����0.5g������X��ֵӦ����0.3g��
��2�������ʣ���0.3��Ϊ��������������ʯ��ʯ��̼��Ƶ�����Ϊ2g-0.3g=1.7g��
ʯ��ʯ��̼��Ƶ���������Ϊ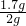 ��100%=85%��
��100%=85%��
��2��һ���Լ���15g�����ַ�Ӧ���ˣ���ĵ������������0.5g���ʷ�Ӧ��̼��Ƶ�������1.5g
������CaCl2������Ϊx������CO2������Ϊy��
CaCO3+2HCl=CaCl2+H2O+CO2��
100 111 44
1.5g x y
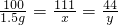
x=1.665g y=0.66g
������Һ���Ȼ��Ƶ���������Ϊ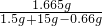 ��100%��10.5%
��100%��10.5%
�ʴ�Ϊ����1��0.3����2��ʯ��ʯ��Ʒ��̼��Ƶ���������Ϊ85%����2����Ӧ������Һ���Ȼ��Ƶ���������Ϊ10.5%��
��������1�����ݷ�Ӧ���������ص����ݽ��з��������Է���5g�����ܹ�����0.5g̼��ƣ������ʣ���0.3g����̼����е����ʣ��ݴ˷�����ɣ�
��2������ʣ������������ʵ������������ʵ������ɼ����ʯ��ʯ��̼��Ƶ���������̼��Ƶ������ɼ����ʯ��ʯ��̼��Ƶ�����������
��3����̼��Ƶ��������Ը��ݻ�ѧ����ʽ����������Ȼ��Ƶ������Ͷ�����̼�����������������������Һ���Ȼ��Ƶ�����������
�����������ѶȽϴ����˸��ݻ�ѧ����ʽ�ļ��㣬���ݱ������ݷ�����ʯ��ʯ��Ʒ�е�̼�����ȫ��Ӧ��ʱ��ʵ������ȷ�����Ĺؼ����ڣ�
��2�������ʣ���0.3��Ϊ��������������ʯ��ʯ��̼��Ƶ�����Ϊ2g-0.3g=1.7g��
ʯ��ʯ��̼��Ƶ���������Ϊ
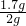 ��100%=85%��
��100%=85%����2��һ���Լ���15g�����ַ�Ӧ���ˣ���ĵ������������0.5g���ʷ�Ӧ��̼��Ƶ�������1.5g
������CaCl2������Ϊx������CO2������Ϊy��
CaCO3+2HCl=CaCl2+H2O+CO2��
100 111 44
1.5g x y
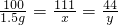
x=1.665g y=0.66g
������Һ���Ȼ��Ƶ���������Ϊ
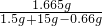 ��100%��10.5%
��100%��10.5%�ʴ�Ϊ����1��0.3����2��ʯ��ʯ��Ʒ��̼��Ƶ���������Ϊ85%����2����Ӧ������Һ���Ȼ��Ƶ���������Ϊ10.5%��
��������1�����ݷ�Ӧ���������ص����ݽ��з��������Է���5g�����ܹ�����0.5g̼��ƣ������ʣ���0.3g����̼����е����ʣ��ݴ˷�����ɣ�
��2������ʣ������������ʵ������������ʵ������ɼ����ʯ��ʯ��̼��Ƶ���������̼��Ƶ������ɼ����ʯ��ʯ��̼��Ƶ�����������
��3����̼��Ƶ��������Ը��ݻ�ѧ����ʽ����������Ȼ��Ƶ������Ͷ�����̼�����������������������Һ���Ȼ��Ƶ�����������
�����������ѶȽϴ����˸��ݻ�ѧ����ʽ�ļ��㣬���ݱ������ݷ�����ʯ��ʯ��Ʒ�е�̼�����ȫ��Ӧ��ʱ��ʵ������ȷ�����Ĺؼ����ڣ�

��ϰ��ϵ�д�
 ��ս�п�����ϵ�д�
��ս�п�����ϵ�д�
�����Ŀ
ij��ѧ��ȤС��Ϊ�˷������������ĺ�����������ʵ���о�����ȡ60g����������������800ϡ�����4�μ��뵽����Ʒ�У�������ݼ�¼���±���

��˵�������������ʲ�����ˮ��Ҳ�������ᷴӦ����
��1����2�β��ʣ���������Ϊ15.2g����ɷ�Ϊ ��
A���� B������̼ C��̼
��2��������������������Ϊ���٣���д��������̣����������С�����һλ��
��3����������ϡ�����������������Ϊ���٣���д��������̣����������С�����һλ����������Ӧ���ɵ��������������ϡ���������Ĺ�ϵͼ��
| ��1�� | ��2�� | ��3�� | ��4�� | |
| ����ϡ����������g�� | 200 | 200 | 200 | 200 |
| ʣ�����������g�� | 37.6 | 15.2 | 4 | 4 |

��˵�������������ʲ�����ˮ��Ҳ�������ᷴӦ����
��1����2�β��ʣ���������Ϊ15.2g����ɷ�Ϊ
A���� B������̼ C��̼
��2��������������������Ϊ���٣���д��������̣����������С�����һλ��
��3����������ϡ�����������������Ϊ���٣���д��������̣����������С�����һλ����������Ӧ���ɵ��������������ϡ���������Ĺ�ϵͼ��
����ij��ʢ��ʯ��ʯ�����к��в�������������ʣ�ij��ѧ��ȤС��Ϊ�˲ⶨʯ��ʯ��̼��Ƶĺ�����ȡ10.0gʯ��ʯ��Ʒ���������ȫ�������ձ��У�������������ϡ���ᣬ�ձ�����ʢ���ʵ�������Ϊ80.0g����Ӧ�����в���ձ�����ʢ���ʵ���������Ӧʱ���¼���±���
�Լ��㣺��1����Ӧ�������ų����ٿ˵Ķ�����̼��
��2����ʯ��ʯ��Ʒ��̼��Ƶ����������Ƕ��٣�
| ��Ӧʱ��/�� | 0 | 2 | 4 | 6 | 8 | 10 |
| �ձ�����ʢ��������/�� | 80.0 | 79.0 | 78.3 | 77.9 | 77.8 | 77.8 |
��2����ʯ��ʯ��Ʒ��̼��Ƶ����������Ƕ��٣�
 ij��ѧ��ȤС��Ϊ�˴��Բⶨһ��ʯ��ʯ��Ʒ��CaCO3��������������Ʋ���������ʵ�飮ʵ��װ����ͼ��ʾ����ȡ��ϸ��2.60gʯ��ʯ��Ʒ����4�μ���ϡ���ᣬ��ַ�Ӧ�����ٲ�������Ϊֹ����÷�Ӧǰ����й����������
ij��ѧ��ȤС��Ϊ�˴��Բⶨһ��ʯ��ʯ��Ʒ��CaCO3��������������Ʋ���������ʵ�飮ʵ��װ����ͼ��ʾ����ȡ��ϸ��2.60gʯ��ʯ��Ʒ����4�μ���ϡ���ᣬ��ַ�Ӧ�����ٲ�������Ϊֹ����÷�Ӧǰ����й����������
