��Ŀ����
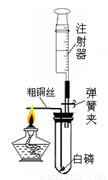
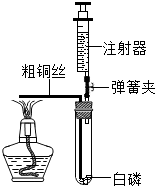
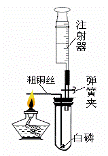
 ij��ѧ��ȤС��Ϊ�˴��Բⶨһ��ʯ��ʯ��Ʒ��CaCO3��������������Ʋ���������ʵ�飮ʵ��װ����ͼ��ʾ����ȡ��ϸ��2.60gʯ��ʯ��Ʒ����4�μ���ϡ���ᣬ��ַ�Ӧ�����ٲ�������Ϊֹ����÷�Ӧǰ����й����������
ij��ѧ��ȤС��Ϊ�˴��Բⶨһ��ʯ��ʯ��Ʒ��CaCO3��������������Ʋ���������ʵ�飮ʵ��װ����ͼ��ʾ����ȡ��ϸ��2.60gʯ��ʯ��Ʒ����4�μ���ϡ���ᣬ��ַ�Ӧ�����ٲ�������Ϊֹ����÷�Ӧǰ����й����������| ��Ӧǰ������װ��+ʯ��ʯ��Ʒ����/g | �����룺ϡ��������/g | ��Ӧ������װ��+��ƿ��ʣ���������/g |
| 104.60 | 20.00 | 123.72 |
��2����������ʵ�������ʯ��ʯ��Ʒ��������Һ��������������ͼ��װ���жϣ���ʵ��������
��������1�����������غ㶨�ɿ���֪������Ӧǰ����ٵļ��ٵ�������Ϊ���ɵĶ�����̼����������϶�����̼��������Ϸ�Ӧ�Ļ�ѧ����ʽ���Լ����̼��Ƶ��������������̼��Ƶ������������ɣ�
��2��������̼������ˮ�У��Ӷ�ʹ��õ���������ƫС�����Ծݴ˽����⣮
��2��������̼������ˮ�У��Ӷ�ʹ��õ���������ƫС�����Ծݴ˽����⣮
����⣺��1�����ɶ�����̼������Ϊ��104.60g+20.00g-123.72g=0.88g
��̼��Ƶ�����Ϊx
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 44
x 0.88g
=
��ã�x=2.00g
����ʯ��ʯ��Ʒ��CaCO3����������Ϊ��
��100%=76.9%
��ʯ��ʯ��Ʒ��CaCO3����������Ϊ76.9%��
��2��������̼������ˮ������������û����ɢ�������Ӷ�ʹ��õ���������ƫС��
�ʴ�Ϊ����1��76.9%��
��2��ƫС��������̼������ˮ������������û����ɢ������
��̼��Ƶ�����Ϊx
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 44
x 0.88g
| 100 |
| x |
| 44 |
| 0.88g |
��ã�x=2.00g
����ʯ��ʯ��Ʒ��CaCO3����������Ϊ��
| 2.00g |
| 2.60g |
��ʯ��ʯ��Ʒ��CaCO3����������Ϊ76.9%��
��2��������̼������ˮ������������û����ɢ�������Ӷ�ʹ��õ���������ƫС��
�ʴ�Ϊ����1��76.9%��
��2��ƫС��������̼������ˮ������������û����ɢ������
�����������ѶȲ������ո��ݻ�ѧ����ʽ�ļ��㼴����ȷ����⣬���������غ㶨�ɼ����������̼����������ȷ������ǰ��ؼ���

��ϰ��ϵ�д�
 ���ٴ���������ѧϰ����ѧ�ں����ν�ϵ�д�
���ٴ���������ѧϰ����ѧ�ں����ν�ϵ�д�
�����Ŀ
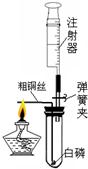
 ע������һ����ͨ��ҽ����е�������ڻ�ѧʵ���з�����Խ��Խ������ã�ij��ѧ��ȤС��Ϊ�˲ⶨ�����������ĺ���������������̽�����
ע������һ����ͨ��ҽ����е�������ڻ�ѧʵ���з�����Խ��Խ������ã�ij��ѧ��ȤС��Ϊ�˲ⶨ�����������ĺ���������������̽�����