��Ŀ����
�á������Ƽ���ƵõĴ����г������Ȼ��Ƶ����ʣ���ѧ��ȤС������ijƷ�ƴ�����Ʒ��̼���Ƶ�������������ʵ��̽��������ʦ��ָ���£�������ͼ��ʾʵ��װ�ã�֧��������ȥ�����Լ���ͨ���ⶨ��Ʒ��ϡ���ᷴӦ������CO2���������������Na2CO3������������װ�����������ã���������Ļӷ�����ÿ����Ӧ�����ö�����ȫ�ģ���ʯ���Ǹ��������Ҫ�ɷ����ռ����ʯ�ң���
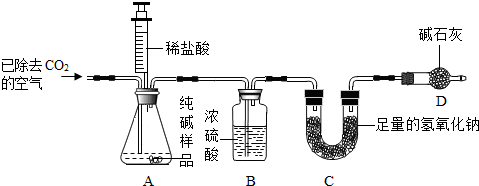
��1����ֹˮ��K���ȶ�װ��A��B�������ӣ�ͨ���ѳ�ȥCO2�Ŀ���һ��������ž�װ��A��B�к��е� ���ٽ���װ��C��D��
��2���ر�ֹˮ��K������������ϡ���ᣨ���ʲ������ᷴӦ����װ��A����Ʒ��������Ļ�ѧ����ʽΪ ��
��3����װ��A�еķ�Ӧ��������һ�δ�ֹˮ��K��������װ��ͨ���ѳ�ȥCO2�Ŀ���һ��������� ���ɣ�װ�� �����ţ��ڷ�Ӧǰ�����������Dz���CO2���������ɴ˼��������Ʒ��Na2CO2������������C�з�����Ӧ�Ļ�ѧ����ʽΪ ����û��װ��D������ʹ�ⶨ��� ���ƫ��ƫС������

��1����ֹˮ��K���ȶ�װ��A��B�������ӣ�ͨ���ѳ�ȥCO2�Ŀ���һ��������ž�װ��A��B�к��е�
��2���ر�ֹˮ��K������������ϡ���ᣨ���ʲ������ᷴӦ����װ��A����Ʒ��������Ļ�ѧ����ʽΪ
��3����װ��A�еķ�Ӧ��������һ�δ�ֹˮ��K��������װ��ͨ���ѳ�ȥCO2�Ŀ���һ���������
���㣺ʵ��̽�����ʵ���ɳɷ��Լ�����,��������ļ�������ӷ���,�εĻ�ѧ����,��д��ѧ����ʽ�����ֱ���ʽ�����뷽��ʽ
ר�⣺��ѧ̽��
��������1�����ݿ����к��ж�����̼���з�����
��2�����������̼���Ʒ�Ӧ�����Ȼ��ơ�ˮ�Ͷ�����̼���з�����
��3�����������غ㶨�ɿ�֪��װ��C�з�Ӧǰ�����������Dz���CO2���������з�����
��2�����������̼���Ʒ�Ӧ�����Ȼ��ơ�ˮ�Ͷ�����̼���з�����
��3�����������غ㶨�ɿ�֪��װ��C�з�Ӧǰ�����������Dz���CO2���������з�����
����⣺��1�������к��ж�����̼�����Դ�ֹˮ��K���ȶ�װ��A��B�������ӣ�ͨ���ѳ�ȥCO2�Ŀ���һ��������ž�װ��A��B�к��еĶ�����̼���ٽ���װ��C��D��
��2�������̼���Ʒ�Ӧ�����Ȼ��ơ�ˮ�Ͷ�����̼����ѧ����ʽΪ��Na2CO3+2HCl�T2NaCl+H2O+CO2����
��3���������غ㶨�ɿ�֪װ��C�з�Ӧǰ�����������Dz���CO2���������������ƺͶ�����̼��Ӧ����̼���ƺ�ˮ����ѧ����ʽΪ��CO2+2NaOH=Na2CO3+H2O��װ��D�������Ƿ�ֹ�����еĶ�����̼��ˮ��������װ��C��������û��װ��D������ʹ�ⶨ���ƫ��
�ʴ�Ϊ����1��������̼��
��2��Na2CO3+2HCl�T2NaCl+H2O+CO2����
��3�������غ㣬CO2+2NaOH=Na2CO3+H2O��ƫ��
��2�������̼���Ʒ�Ӧ�����Ȼ��ơ�ˮ�Ͷ�����̼����ѧ����ʽΪ��Na2CO3+2HCl�T2NaCl+H2O+CO2����
��3���������غ㶨�ɿ�֪װ��C�з�Ӧǰ�����������Dz���CO2���������������ƺͶ�����̼��Ӧ����̼���ƺ�ˮ����ѧ����ʽΪ��CO2+2NaOH=Na2CO3+H2O��װ��D�������Ƿ�ֹ�����еĶ�����̼��ˮ��������װ��C��������û��װ��D������ʹ�ⶨ���ƫ��
�ʴ�Ϊ����1��������̼��
��2��Na2CO3+2HCl�T2NaCl+H2O+CO2����
��3�������غ㣬CO2+2NaOH=Na2CO3+H2O��ƫ��
���������⽫�����ʵ���л��ؽ���������п��飬ͨ���������⣬���������Ѹ���һϵ�е���ʾ��������������⣬�������õ���Ϣ����ַ������룬�����е�֪ʶ������ϵ��Ȼ���������Ƶ����Ӷ��ﵽ��������Ŀ�ģ�

��ϰ��ϵ�д�
�����Ŀ
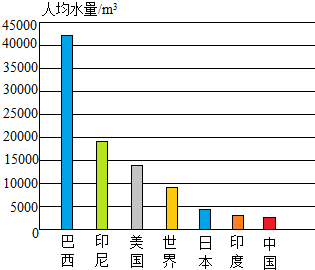 ��ͼ��ʾΪ�����˾�ˮ����һЩ���ҵ��˾�ˮ����
��ͼ��ʾΪ�����˾�ˮ����һЩ���ҵ��˾�ˮ����
 ��ͼ��ij��θҩ�IJ��ֱ�ʶ��θҩ���������������к�θ������θ�ᣮij���߰���ʶ�ϵķ��÷�����ҩ����ҩ�����ת�����㣺
��ͼ��ij��θҩ�IJ��ֱ�ʶ��θҩ���������������к�θ������θ�ᣮij���߰���ʶ�ϵķ��÷�����ҩ����ҩ�����ת�����㣺