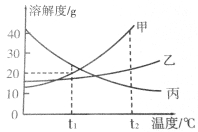
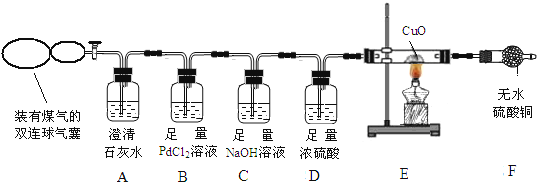
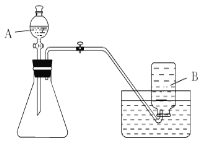
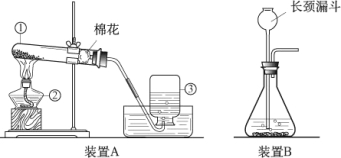
��Ŀ����
����Ŀ����ͼ��ij��Ƭ��˵���飬���Ķ�����������⡣
X X �� Ƭ ��Ҫ�ɷ֣����������C6H11O72Ca ҩƷ���2.5gÿƬ��C6H11O72Ca 0.2g �� ����ÿ��2�Σ�ÿ��һƬ |
������������к���_________��Ԫ�ء�
�������������̼��Ƶ���������_______��
����˵������ô˸�Ƭ��ÿ�첹��Ƶ�Ϊ�� ����ֻд��ʽ������������
�����֪��C6H11O72Ca ����Է�������Ϊ 430
��2�����з����� 4.9t���������� H2SO4 ����������Ϊ20���������ķ���м��Ӧ��������FeSO4�������Ƕ��٣�
���𰸡� ��1����1������(1��) ��72��40 �� 9��5( 1��)���������Ҳ�ɣ� ��2��0.2g����40/430��100����1 �ֺ������ɵ÷֣�2��1.52t
��������
�����������1����������������ƵĻ�ѧʽ��֪�京�� ����Ԫ�أ�
�������������̼��Ƶ��������ǣ�6��12:40=72��40 �� 9��5( 1��)���������Ҳ�ɣ�
����˵������ô˸�Ƭ��ÿ�첹��Ƶ�Ϊ��2��0.2g����40/430��100����1 �ֺ������ɵ÷�
��2����: ������ FeSO4 ������Ϊ x��
Fe +H2SO4![]() FeSO4+ H2�� ��������������������1 ��
FeSO4+ H2�� ��������������������1 ��
98 152
4.9t��20�� x
98��152= 4.9t��20����x ��������������������1 ��
x =1.52t ��������������������1 ��
������ FeSO4 ������Ϊ 1.52t��

 �����̸�Ӯ����ٸ�Ч�����ܸ�ϰ���ϿƼ�������ϵ�д�
�����̸�Ӯ����ٸ�Ч�����ܸ�ϰ���ϿƼ�������ϵ�д� �����ҵ�����������ѧ���ӳ�����ϵ�д�
�����ҵ�����������ѧ���ӳ�����ϵ�д� ����ѧ��Ӯ�����ϵ�д�
����ѧ��Ӯ�����ϵ�д�����Ŀ����Ba(OH)2��Һ�ⶨij�ֵ�����(NH4)2SO4����������(��������ˮ�������μӷ�Ӧ)��
ʵ�����£���ȡ�õ���20g��ˮ��ȫ�ܽ⣬�����Һ�в��ϵ���Ba(OH)2��Һ������
���弰�������������Ba(OH)2��Һ��������ϵ������ʾ��
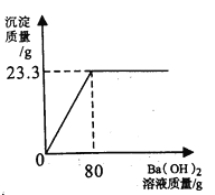
ʵ����� | 1 | 2 | 3 |
����Ba(OH)2��Һ������/g | 20 | 40 | 60 |
�������������/g | 0��85 | m | 2��55 |
�Է��������㣺[��֪��(NH4)2SO4+Ba(OH)2=BaSO4��+2H2O+2NH3��]
��1��������m��ֵΪ g��
��2��ʵ����������������Һ�����ʵ�����������
��3)�õ�����(NH4)2SO4����������������������ȷ��0��1%��