��Ŀ����
����Ŀ��ú���ۺ������ǽ�ú����������ǿ�ȣ�ʹú�ֽ���������õ����ʣ�ú��������һ�֡�ú������Ҫ�ɷ���ʲô�أ�ij��ȤС��Ϊ��չ����̽����
���������ϡ� ��1�� ú���п��ܺ��� CO��CO2��H2��CH4 �е�һ�ֻ��֡�
��2�� �����£��Ȼ���(PdCl2)��Һ���� CO ʱ�����Ļ�ѧ��Ӧ����ʽΪ��
CO ��PdCl2��H2O ![]() CO2��Pd��(��ɫ) ��2HCl
CO2��Pd��(��ɫ) ��2HCl
��ʵ�鷽������ȤС���ͬѧ�������װ��̽��ú���еijɷ֡�
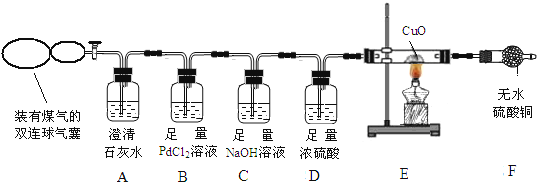
���������ۡ���ش�ʵ���е��й����⣺
��1��ʵ�鿪ʼ��A ������������˵��ú���в�����____________________��
��2�� ��ú������ CO ���ڣ��� B�в����������� ��
��3�� д�� C �з�����Ӧ�Ļ�ѧ����ʽ___________________________����дһ�����ɣ�
D ��Ũ�����������_________________________��
��4�� �� F ����ˮCuSO4����ɫ����E��һ�������Ļ�ѧ����ʽΪ ��
��5����ȼFװ�õ��������壬�л��������������Ϊú���к� CH4��������CH4����ȼ�գ�CH4 ȼ�յĻ�ѧ����ʽ��____________________________________����ʦ����Ϊ����ȷ��ú���к��� CH4��������__________________________��Ϊ��ȷ��ú�����Ƿ���CH4����ʦ���Ų�ȡ�IJ��������� ���۲쵽��������___________________________________���ɴ���֤ú����һ������CH4��
���𰸡� ���������ۡ� ��1��CO2��2����Һ�в�����ɫ������3��NaOH+ HCl ![]() NaCl +H2O �� 2NaOH+ CO2
NaCl +H2O �� 2NaOH+ CO2 ![]() Na2CO3 +H2O ����������ȥ�����е�ˮ�֣�4��H2 +CuO
Na2CO3 +H2O ����������ȥ�����е�ˮ�֣�4��H2 +CuO![]() Cu +H2O ��5��CH4 +2O2
Cu +H2O ��5��CH4 +2O2 ![]() CO2 +2H2O ����ȷ��H2��Eװ���е� CuO �Ƿ���ȫ��Ӧ��F�����������п����� H2 ��ش������ 2 ��(�����𰸼��ɵ÷� ) �ڻ����Ϸ���һ���ڱڸ��г���ʯ��ˮ���ձ�(�����𰸼��ɵ÷�) �ձ��ڱڳ���ʯ��ˮ�����(����һ���ɫ����)
CO2 +2H2O ����ȷ��H2��Eװ���е� CuO �Ƿ���ȫ��Ӧ��F�����������п����� H2 ��ش������ 2 ��(�����𰸼��ɵ÷� ) �ڻ����Ϸ���һ���ڱڸ��г���ʯ��ˮ���ձ�(�����𰸼��ɵ÷�) �ձ��ڱڳ���ʯ��ˮ�����(����һ���ɫ����)
��������
�����������1��ʵ�鿪ʼ��A ������������˵��ú���в����ڶ�����̼����2�� ��ú������ CO ���ڣ�����һ����̼�����ʾ��л�ԭ�Լ����Ͽ�֪�� B�в�������������Һ�в�����ɫ��������3�� д�� C �з�����Ӧ�Ļ�ѧ����ʽNaOH+ HCl ![]() NaCl +H2O �� 2NaOH+ CO2
NaCl +H2O �� 2NaOH+ CO2 ![]() Na2CO3 +H2O ��D ��Ũ����������Ǹ���������ȥ�����е�ˮ�� ����4�� �� F ����ˮCuSO4����ɫ��˵����ˮ���ɣ���E��һ�������Ļ�ѧ����ʽΪH2 +CuO
Na2CO3 +H2O ��D ��Ũ����������Ǹ���������ȥ�����е�ˮ�� ����4�� �� F ����ˮCuSO4����ɫ��˵����ˮ���ɣ���E��һ�������Ļ�ѧ����ʽΪH2 +CuO![]() Cu +H2O����5����ȼFװ�õ��������壬�л��������������Ϊú���к� CH4��������CH4����ȼ�գ�CH4 ȼ�յĻ�ѧ����ʽ��CH4 +2O2
Cu +H2O����5����ȼFװ�õ��������壬�л��������������Ϊú���к� CH4��������CH4����ȼ�գ�CH4 ȼ�յĻ�ѧ����ʽ��CH4 +2O2 ![]() CO2 +2H2O������ȷ��ú���к��� CH4��������H2��Eװ���е� CuO �Ƿ���ȫ��Ӧ��F�����������п����� H2Ϊ��ȷ��ú�����Ƿ���CH4����ʦ���Ų�ȡ�IJ��������ڻ����Ϸ���һ���ڱڸ��г���ʯ��ˮ���ձ����۲쵽���������ձ��ڱڳ���ʯ��ˮ����ǣ��ɴ���֤ú����һ������CH4��
CO2 +2H2O������ȷ��ú���к��� CH4��������H2��Eװ���е� CuO �Ƿ���ȫ��Ӧ��F�����������п����� H2Ϊ��ȷ��ú�����Ƿ���CH4����ʦ���Ų�ȡ�IJ��������ڻ����Ϸ���һ���ڱڸ��г���ʯ��ˮ���ձ����۲쵽���������ձ��ڱڳ���ʯ��ˮ����ǣ��ɴ���֤ú����һ������CH4��

����Ŀ����2017ǭ���ϣ�������ƿ��ɫ��ʧȥ��ǩ��̼������Һ������������Һ����μ����䷽���ܶࡣ
��������⣩���ṩ����������Һ��ϡ���ᡣ�ܷ�̼������Һ������������Һ��������
��������裩��ʧȥ��ǩ��������Һ�ֱ���ΪA��B������AΪ̼������Һ��BΪ����������Һ��
�����˼·�����мס�����λͬѧ�ֱ�˼���ó����³�������˼·��
�ף���������Һȡ�����ֱ����ϡ���ᣬ�����Ƿ������ݲ������жϸ���������Һ��
�ң���������Һȡ�����ֱ��������������Һ���������Ƿ��а�ɫ�����������жϸ���������Һ��
��1����������˼·�Ƿ���ȷ______����������ȷ������һ����ȷһ������ȷ������
��ʵ��̽�������������ʵ����̣�
��2����������ʵ��˼·��ѡ��һ������Ϊ��ȷ��˼·___������������������������ʵ�顣
��3����д��ʵ�鱨�棺
ʵ�鲽�� | ʵ������ | ���ۣ���ѧ��Ӧ����ʽ |
�ٽ�A��B����Һ��ȡ�������ֱ������֧�Թ��У���_ | A��Һ��Ʒ����____ | ��AΪ___��Һ��BΪ__��Һ����Ӧ�Ļ�ѧ����ʽΪ____�� |
����ɢ˼ά������̼������Һ������������Һ�������Դ������Լ���ѡȡ������ѡ��һ���ܼ�����������Һ���Լ�____������д��ţ���
A KOH B Na2SO4 C BaCl2 D NaCl
����Ŀ����ͼ��ij��Ƭ��˵���飬���Ķ�����������⡣
X X �� Ƭ ��Ҫ�ɷ֣����������C6H11O72Ca ҩƷ���2.5gÿƬ��C6H11O72Ca 0.2g �� ����ÿ��2�Σ�ÿ��һƬ |
������������к���_________��Ԫ�ء�
�������������̼��Ƶ���������_______��
����˵������ô˸�Ƭ��ÿ�첹��Ƶ�Ϊ�� ����ֻд��ʽ������������
�����֪��C6H11O72Ca ����Է�������Ϊ 430
��2�����з����� 4.9t���������� H2SO4 ����������Ϊ20���������ķ���м��Ӧ��������FeSO4�������Ƕ��٣�
����Ŀ����ͼ�У���Բ�ס��ҡ��������ֱ��ʾһ����Һ����Բ���ཻ����Ϊ����Һ��Ϻ���ֵ���Ҫʵ�������±��в�����ͼʾ��ϵ����
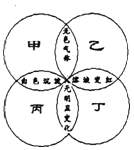
�� | �� | �� | �� | |
A | Na2CO3 | H2SO4 | BaCl2 | ��ɫʯ�� |
B | (NH4)2SO4 | NaOH | Ba(NO3)2 | ��ɫ��̪ |
C | K2CO3 | HCl | Ba(OH)2 | ��ɫʯ�� |
D | HCl | Na2CO3 | AgNO3 | ��ɫ��̪ |