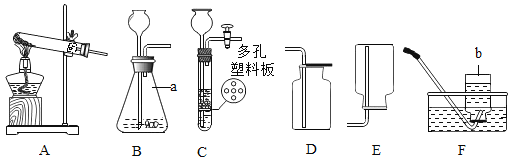
��Ŀ����
����Ŀ��ˮ������֮Դ������ᾭ�÷�չ����ȱ�ٺͲ����������Ҫ��Ȼ��Դ�ͻ���Ҫ�ء�
��1��2018��3��22���ǵڶ�ʮ�Ľ조����ˮ�ա���Ϊ��Ӧ��һ���⣬ij��ѧ����С���ͬѧ��ϻ������ţ����ڶ����д�������ˮ����ȡ�����ԡ������ij�ղɼ���ˮ�����ش��������⣺
��С��ͬѧȡ������������ˮ��Ʒ���۲쵽��ˮ������ɫ���������Լ�������________��������ȥˮ���е���ζ��ɫ�ء�
��Ϊ�˱�����������ˮ�ʣ�������������������_________������ţ���
Aȫ�����ƺ��е�ֲ������ B�����ˮ�ŷŹ�ҵ��ˮ
C��ֹ����������������� D���ڶԺ�ˮˮ�ʽ��м��
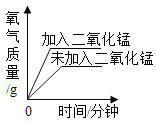
�ۻ���С���ͬѧ����pH��ֽ���Բⶨ������ˮ�ʵ������ǿ������д������IJ���������___________��
��2����ͼ��ʾΪˮ����Ȼѭ����һ���֡�
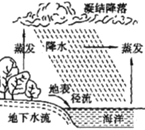
�ٺ�ˮ����_____���������������������������
�����з����ܴӺ�ˮ�л�ȡ��ˮ��Դ����__________��
A���� B���� C���� D����
��2��ˮ����Һ�����������������������ش�����塣
�������У�������________����Ӳˮ����ˮ��ͨ��______�ķ�����Ӳˮ������
�������Ȼ��ƹ����ˮ����0.9%��������ˮ����ͼ��ʾΪ��ص�ʵ��������裺
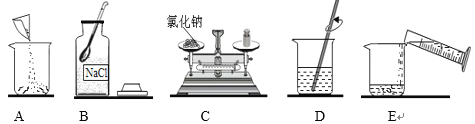
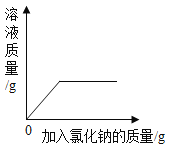
����0.9%������ˮ����ȷ����˳����_______������ĸ��ţ���������Ͳ��ȡˮʱ���Ӷ�����������������ȷ��������������ˮ��������������_______0.9% �������������������=����
���𰸡�����̿ A �ڲ���Ƭ��״ɰ��Ϸ�һƬpH��ֽ���ò�����պȡ������ˮ�ε���ֽ�ϣ�����ֽ��ʾ����ɫ�����ɫ���Ƚϣ����ɵó�������ˮ��pH ����� B ����ˮ ��� BCAED ��
��������
�⣺��1�������ڻ���̿���������ԣ�С��ͬѧȡ������������ˮ��Ʒ���۲쵽��ˮ������ɫ���������Լ�����������̿��������ȥˮ���е���ζ��ɫ�أ�
��A ȫ�����ƺ��е�ֲ�����������ƻ���������ˮ�ʣ��ʴ���
B ��������ŷŹ�ҵ��ˮ���ɱ�����������ˮ�ʣ�����ȷ��
C ��ֹ��������������������ɱ�����������ˮ�ʣ�����ȷ��
D ���ڶԺ�ˮˮ�ʽ��м�⣬�ɱ�����������ˮ�ʣ�����ȷ��
�ۻ���С���ͬѧ����pH��ֽ���Բⶨ������ˮ�ʵ������ǿ��������IJ��������ǣ��ڲ���Ƭ��״ɰ��Ϸ�һƬpH��ֽ���ò�����պȡ������ˮ�ε���ֽ�ϣ�����ֽ��ʾ����ɫ�����ɫ���Ƚϣ����ɵó�������ˮ��pH��
��2���ٺ�ˮ�к����Ȼ��ơ��Ȼ��Ƶȣ����ڻ���
������õ���ˮ�Ǵ�ˮ���ܴӺ�ˮ�л�ȡ��ˮ��Դ��
��3���������У������÷���ˮ����Ӳˮ����ˮ��������ˮ������ĭ�ٵ���Ӳˮ��������ˮ������ĭ�������ˮ��ͨ����еķ�����Ӳˮ������
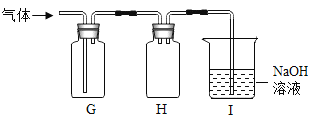
������500g���ʵ���������Ϊ10%��NaCl��Һ�����ȼ���������Һ�����Ȼ��ƺ�ˮ���������ٳ���������Ȼ��ƺ���ȡˮ���������ܽ⣬����������Һ����ȷ����˳��ΪBCAED��������Ͳ��ȡˮʱ���Ӷ�����ʵ����ȡ��ˮƫ�࣬������������ȷ��������������ˮ����������������0.9%��

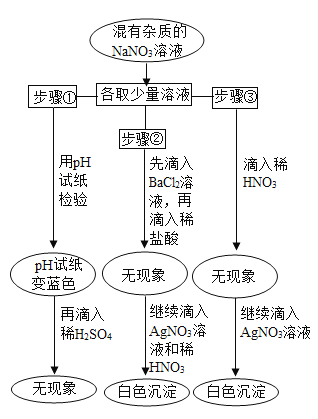
����Ŀ��ʵ������һƿ��ǩ��ȱ����ƿ��û����ȫ�ܷ����ɫ��Һ����ѧС���ͬѧ����ѯ����ʦ��ֻ֪����NaCl��NaOH��Na2CO3��NaHCO3�е�һ����Һ��Ϊȷ��ԭƿ���Ǻ������ʲ��ж����Ƿ���ʣ���ѧС���ͬѧ���������µ�̽�����
�����в��룩
��������ԭ��Һ��������NaCl�� ��������ԭ��Һ��������NaOH��
��������ԭ��Һ��������Na2CO3�� ��������ԭ��Һ��������NaHCO3��
��ǩ�����ȱ��ԭ����________��
���������ϣ������£��й����ʵ������Ϣ�����
���� | NaCl | NaOH | Na2CO3 | NaHCO3 |
�����µ��ܽ��/g | 36 | 109 | 21.5 | 9.6 |
������ϡ��Һ��pH | 7 | 13 | 11 | 9 |
�����ʵ������Ϣ��֪��ԭ��Һ������һ������________��
������ʵ�飩
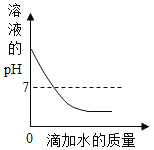
��1��ȡ������Ʒ�������Һ��pH����7����ԭ��Һ�����ʿ϶�����________��
��2��ͬѧ����ȡ��Ʒ�ֽ���������ʵ�飬ʵ��������£�
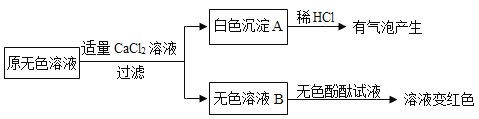
�����ɰ�ɫ����A�Ļ�ѧ����ʽ________��
��ͨ��ʵ������ж���Ʒ��ɫ��Һ�к��е�������________��
����ý��ۣ�
ʵ����ɺ����յõ��Ľ��ۣ�ԭƿ�е�������________�����ѱ��ʡ�